Ch. 2.1 Properties of Matter
advertisement

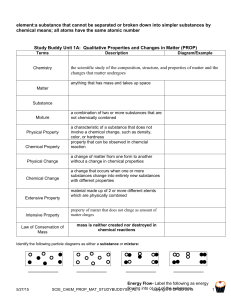
Ch. 2.1 Properties of Matter Classified as either extensive or intensive. Extensive properties—depend on the amount of matter in a substance…mass, volume. Intensive properties—independent of mass and volume…strength, hardness, ductility, etc. Identifying Substances. Intensive properties are similar among substances with the same composition. Physical properties—those that can be measured or observed without changing the substance’s composition. States of Matter Solids—have definite shape and volume. Almost incompressible; expand slightly when heated. Liquids—indefinite shape, constant volume. Almost incompressible; expand slightly when heated. Gases—take the shape and volume of their container. Easily compressed. Vapor—gaseous state of a substance that is generally liquid or solid at room temperature. Physical Changes Some properties of a material change, but the substance’s composition does not change. Physical changes can be either reversible (boiling or freezing) or irreversible (cutting, grinding).