Study Guide 2.1 Properties of Matter

Study Guide 2.1 Properties of Matter
Everything is made of matter whether biotic (living) or abiotic (non-living).
Characteristic properties of matter are the same no matter what the size or shape.
When a substance goes through a physical change or change in appearance, its chemical make-up and chemical properties do not change.
Many substances share characteristic properties like melting point, texture, etc. so you may need to study 2 or 3 characteristics to accurately identify a substance.
Possible Essay: Explain the difference between atoms and molecules.
Atoms are the smallest particles of an element. Atoms can combine with other atoms to form molecules. A molecule is a group of atoms that are joined together and act as a single unit.
Molecules can vary in size from just two atoms to billions of atoms. Atoms within a molecule are held together by chemical bonds.
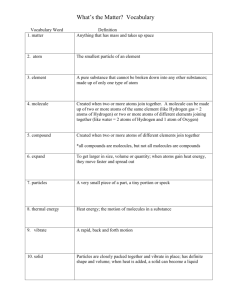
Vocabulary. Be able to define and give examples of each. See your notes.
Matter
Atom
Element
Molecule
Compound
Chemical bonds
Mixture
Solution
Pure substance
Characteristic property
Physical property
Chemical property
Physical change
Chemical change
States of Matter: solids, liquids, and gases. o What they look like, volume, shape, how they move (use thermal energy in moving)
Review P21 1-5 Questions and answers.
Review Video Quiz on Properties of Matter
Be able to determine if something is undergoing a physical or a chemical change .
Be able to define and understand the following terms: See your notes.
Mass o Use a triple beam balance, Units: grams or kilograms
Weight o Use a scale, Units: pounds
HINT: Know the difference between your weight on Earth and your weight on the moon and your mass on Earth and your mass on the moon (Essay).
Volume o Solids, cubes – use a metric ruler to measure length x width x height, Units: cm 3 o Solids, irregular shape (ex. marble) - use a graduated cylinder, Units: mL o
Liquids – use a graduated cylinder, Units: milliliters, liters
Density o Mass (g) divided by Volume (mL)
Study Guide 2.1 Properties of Matter
o Understand that when something is more dense than water (or whatever it is put in), it will sink. If it is less dense, it will float.