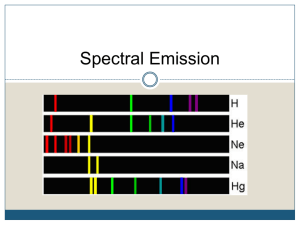
Chapter 4: Spectroscopy
advertisement

Chapter 4: Spectroscopy • What is spectroscopy? • Characteristics of spectra – continuous, emission, absorption – Kirchoff’s law • Structure of the atom – Bohr model • Transitions and spectra – atoms, molecules • Astronomical information from spectra – Fraunhofer lines – wavelengths, intensity, broadening Information from Spectra Almost all that we know about planets, stars, and galaxies is obtained from studies of the light received from them. Spectral Lines • Newton: –sunlight through pinhole to prism –spectrum = continuous rainbow • Blackbody radiation: –radiation at all wavelengths –spectrum = continuous rainbow Blackbody Radiation • Consider an idealized object that absorbs all the electromagnetic radiation that falls on it - called a “blackbody.” • A blackbody will absorb all energy incident on it and heat up until it is emitting energy at the same rate that it absorbs energy. • The equilibrium temperature reached is a function of the total energy striking the blackbody each second. Characteristics of Blackbody Radiation • Three characteristics of a blackbody : 1. A blackbody with a temperature higher than absolute zero emits some energy at all frequencies (or wavelengths). 2. A blackbody at higher temperature emits more energy at all frequencies (or wavelengths) than does a cooler one. 3. The higher the temperature of a blackbody, the higher the frequency (the shorter the wavelength) at which the maximum energy is emitted. Blackbody Radiation • Blackbody radiation: the distribution of radiation emitted by any heated object. • The curve peaks at a single, well-defined frequency and falls off to lesser values above and below that frequency. The overall shape (intensity vs frequency) is characteristic of the radiation emitted by any object, regardless of its size, shape, composition, or temperature. Planck Spectrum • As an object is heated, the radiation it emits peaks at higher and higher frequencies. • Shown here are curves corresponding to temperatures of 300 K (room temperature), 1000 K (begin to glow deep red) 4000 K (red hot), and 7000 K (white hot). Spectroscope Spectral Lines from Sunlight Wollaston (1802) – sunlight through slit to prism – spectrum = rainbow with holes Fraunhofer (~1812) – cataloged over 600 dark lines Another Type of Spectra • Further study revealed that if gases are heated until they emit light, neither a continuous nor a spectrum with dark lines is produced. – A spectrum made up of bright lines appears. • Also, each element was discovered to produce its own distinctive pattern of bright lines. – Used as a way to identify the composition of an unknown substance. Continuous Spectra Radiation is distributed over all frequencies, not just a few specific frequency ranges. Emission Spectra Pattern of bright spectral lines produced by an element. Absorption Spectra Pattern of dark spectral lines where light within a number of narrow frequency ranges has been removed. When are Each of the Three Types of Spectra Observed? • The situation in which each of the three types of spectra is observed was summarized in a set of rules by Gustav Kirchoff in the 1860’s. • These rules are known as “Kirchoff’s laws”. Kirchoff’s Laws • 1st law: A luminous solid or liquid, or a sufficiently dense gas, emits light of all wavelengths and produces a continuous spectrum of radiation. • 2nd law: A low-density hot gas emits light whose spectrum consists of a series of bright emission lines which are characteristic of the chemical composition of the gas. • 3rd law: A cool thin gas absorbs certain wavelengths from a continuous spectrum, leaving dark absorption lines in their place superimposed on the continuous spectrum. Observed Spectra and Background Type of spectrum seen depends on the temperature of the thin gas relative to the background temperature. TOP: thin gas cooler than background, absorption lines seen. BOTTOM: thin gas hotter than background, emission lines seen. How are Spectral Lines Created? • In the last chapter, stated that – electromagnetic waves are created by moving charges and – all matter is composed of atoms, which are in turn composed of • protons (+ charge), • electrons (- charge), and • neutrons (neutral charge) • Is there a connection between composition of matter and the spectral lines produced? Structure of the Atom • For at least 25 centuries, matter believed to be made of tiny particles -- atoms. • Newton thought that atoms were hard and indivisible. • Complex structure of the atom not observed until 20th century. –In 1897, J.J. Thomson discovered the electron. • plum pudding model –In 1911, Ernest Rutherford detected atomic nucleus. • alpha particles shot at thin gold foil Rutherford Model of the Atom • Early models of the atom are like a miniature solar system with the electrons orbiting the nucleus, just as the planets orbit the Sun. • The electrons must be in motion. (e and p attract each other; stationary e would fall into nucleus.) • Most of the atom is empty space. • Problem: Since e are in motion, why don’t atoms emit E-M radiation continuously? Why don’t e lose energy and spiral into nucleus? Bohr Model of the Atom • “Planetary model” of the atom. – Neutrons and protons occupy a dense central region called the “nucleus”. – Electrons orbit the nucleus much like planets orbiting the Sun. • Modifications – Only certain select radii are possible for the electron orbits. – If an electron moves in an allowed orbit, it radiates no energy. – The amount of energy required to move from one orbit to another is fixed. E Photons E • Electrons may exist only in orbitals having certain specified energies. • Atoms can absorb only specific amounts of energy as their electrons are boosted to excited states; atoms emit only specific amounts of energy when their electrons fall back down to lower energy states. • The light absorbed or emitted must be in “packets” of electromagnetic radiation containing a specific amount of energy. • These packets are called “PHOTONS.” • The energy of a photon is related to the frequency of the electromagnetic energy absorbed or emitted. Frequency and Energy The energy of a photon is related to the frequency of light emitted or absorbed by E = hf where h = Planck’s constant Recall that wave speed relates frequency and wavelength: v = f and for light, so, E f c = f or E 1/ “Every physicist thinks he knows what a photon is. I spent my life to find out what a photon is and I still don’t know.” Albert Einstein Emission Electrons drop to lower energy levels releasing energy in the form of EMR Hydrogen Atom • The energy of particles in Bohr atom are restricted to certain discrete values. • The energy is quantized. – only certain orbits with certain radii are allowed; – orbits in between simply don't exist. • Energy levels labeled by an integer n - called a quantum number. • The lowest energy state is generally termed the ground state. • The states with successively more energy than the ground state are called the first excited state, second excited state, etc. Terminology • The “normal” condition of an atom is called the ground state. – minimum energy configuration of the atom • If an orbiting electron is given enough energy to escape the atom, the atom is said to be ionized. • Between ground state and ionization, the electron can only exist in certain well-defined excited states. – Each excited state has a specific energy (quantized). – Electrons moving from one energy level to another, absorb or emit an amount of energy equal to the difference between the energy levels. – The energy is absorbed or emitted in the form of a photon. – The energy of the photon is proportional to frequency. Excitation and Emission • If an atom is given energy, electron may jump to a more distant orbit. • Atoms do not stay in this energized state long. • Electron will fall down to a lower orbit, emitting a photon. Hot Gas Spectra • The visible spectrum of the hot gases in a nearby star-forming region known as the Omega nebula (M17). • Shining by the light of several very hot stars, the nebula produces a complex spectrum of bright and dark lines (bottom). • Spectrum also shown as an intensity trace from red to blue (center). What type of spectra is this (continuous, emission, or absorption)? From Atoms to Molecules If two or more atoms combine to form a molecule, do the electron energy levels and, consequently, the observed spectra change? If so, how much? Molecular Motions Three ways molecules can change to emit or absorb E-M radiation. change electron arrangement (e.g., electron in the outermost orbital of the oxygen atom drops to lower-energy state) change rotational state to a lower energy mode change vibrational state to a lower energy mode Hydrogen Spectra (a) Molecular Hydrogen (b) Atomic Hydrogen Information from Spectra • High temperatures, atoms mainly ionized. – Spectrum is continuous. • Cooler temperatures, more bound electrons making transitions. – Spectral lines help determine chemical composition. • Spectral line strength depends on – the number of a specific type atoms in the gas – the temperature of gas containing atoms • number in each excited state • collisions Spectral Linewidth • May expect narrow, distinct spectral lines. • Physical mechanisms can broaden spectral lines. – Doppler Effect • Thermal motion • Rotation • Gas Turbulence – Collision Broadening – Magnetism Doppler Effect: Thermal Motion of Atoms • Atoms moving randomly. • Redshifted, blueshifted, and unshifted emission lines created with respect to the observer. • In the detector, individual redshifted and blueshifted emission lines merge with the unshifted lines to produce broadened spectral lines. Doppler Effect: Rotation • Rotation of star/gas will produce a broadening of spectral lines. • Photons emitted from side spinning toward us, blueshifted. • Photons emitted from side spinning away from us, redshifted. Observed spectral line is broadened. Doppler Effect: Turbulence • If gas in a cloud is churning in eddies and vortices of different sizes, spectral lines from each of those parts of the cloud will be Doppler-shifted randomly wrt another part of the cloud. • If the cloud is far away or small, light from all parts of the cloud will be blended in the detector. • Overall effect similar to thermal broadening, but NOT related to temperature of the gas. Spectral Linewidth • Physical mechanisms can broaden spectral lines. – Doppler Effect • Thermal motion • Rotation • Gas Turbulence – Collision Broadening – Magnetism Spectral Information from Starlight Observed Spectral Characteristics Information Provided Peak frequency or wavelength (continuous spectra only) Temperature Lines present Composition, Temperature Line intensity Composition, Temperature Line width Temperature, Turbulence, Rotation Speed, Density, Magnetic Field Doppler shift Line of sight velocity What’s important? • spectroscopy definition • spectra: continuous, emission, absorption – How is each of the above formed? – Kirchhoff’s Laws • relationship between type of spectra observed and necessary conditions for that observation. • “fingerprinting” composition • atoms • protons, electrons, neutrons • structure: Bohr model of the atom; quantization • relationship between structure and spectra; photons • spectral line broadening: possible causes • information from spectra