Chemistry Test Review
advertisement

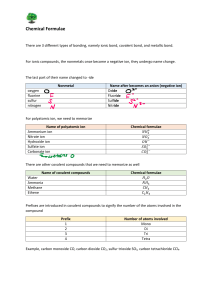
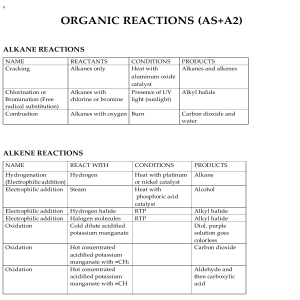
Name _________________ 1. Show how an ion becomes positive and negative. 3. Draw an ionic bond with Na and Cl: 4. Show a covalent bond with Fluorine and Fluorine. 5. Draw the electron shells for Chlorine. Include the number of electrons on each shell. 9. Fe + HCl 10. C2 H4 + 02 Balance the following… FeCl2 + H2 _________________________________________ CO2 + H20 _________________________ 2. Give an example of a polyatomic ion: Chemistry Test Review electron dot diagram valence electrons chemistry chemical equation (draw) chemical formula (draw) subscript reactant product conservation of matter coefficient endothermic exothermic synthesis decomposition plastic monomer Composite replacement ion ionic bond polyatomic ion covalent bond double bond triple bond polar non polar acid base ph scale polymer Give an example of each type of reaction, use chemical equations. 6. Synthesis 7. Replacement 8. Decomposition 11. Write a chemical formula with 2 subscripts: Write the chemical formulas for… 12. Ammonium chloride. (use chart page 185) 13. Lithium oxide 15. Draw the pH scale on the back of this paper. Label 1, 7, 14, acid, base, blue litmus red, red litmus blue, low Hydrogen ions, high Hydrogen ions. 16. Win $1,000,000 on Test review on Mrs. Schmitt’s website.