Oxygenation and Acid-Base Balance - Vanderbilt University Medical
advertisement

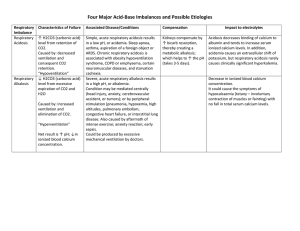
Acid-Base Balance and Imbalance James Barnett, RN, MSN Clinical Educator – Neuroscience PCC Vanderbilt University Medical Center May 2007 Definitions pH Measurement of how alkaline or acid a substance is Chemistry Neutral ph = 7 pH < 7 is acid pH > 7 is alkaline Normal human blood pH is slightly alkaline at 7.35 – 7.45 Definitions An Acid is… A molecule that can donate a H+ ion Examples: H2O H+ + OH H2CO3 H+ + HCO3 An acid can be weak, moderate, or strong depending on its pH Weaker acids are closer to 7 Stronger acids are closer to 1 Definitions A Base or alkali is… A molecule that can accept a H+ ion Examples: H+ + OH- H2O H+ + HCO3- H2CO3 A base can be weak, moderate, or strong depending on its pH Weaker bases are closer to pH 7 Stronger bases are closer to pH 14 An Important Equilibrium Equation H2O + CO2 H2CO3 H+ + HCO3- Water plus carbon dioxide equals carbonic acid which is broken down by carbonic anhydrase into a hydrogen ion and bicarbonate. Types of Acids Volatile acids Easily move from liquid to gas state Within the body Lung can remove H2CO3 + renal enzyme H2O + CO2 (both of which are exhaled) Carbon dioxide is therefore considered an acid KEY CONCEPT: As CO2 rises, there is more acid respiratory acidosis KEY CONCEPT: As CO2 drops, there is less acid respiratory alkalosis Types of Acids Nonvolatile acids Cannot be changed to gas state Within the body Must be removed by the kidneys (metabolic) Examples Keto acids Lactic acids Acid-Base Imbalance Acid Imbalance Too much CO2 leads to decreased pH and an acid condition Too little CO2 leads to increased pH and an alkaline condition PaCO2 Amount of CO2 dissolved in the blood Good indicator of respiratory and ventilatory function Base Imbalance Major base in body is bicarbonate (HCO3-) Regulated by the kidneys (metabolic) Too little HCO3- causes decrease in pH and acid conditions Too much HCO3- causes increase in pH and alkaline conditions More definitions Acidemia – condition where blood is more acid than normal (pH < 7.35) Alkalemia – condition where blood is more alkaline than normal (pH > 7.45) Acidosis – the process causing acidemia Alkalosis – the process causing alkalemia Respiratory Imbalances Respiratory mechanisms involve either Retention of excess carbon dioxide Retention of insufficient carbon dioxide Over- or Under-ventilation or respiration abnormalities are the main causes of this imbalance Metabolic Imbalances Metabolic mechanisms involve Renal function alteration Production of acidic metabolic products Loss of acid from the body The Normal’s pH 7.35 – 7.45 PaCO2 35 – 45 mmHg HCO3- 22 – 26 mEq/L PaO2 80 – 100 mmHg The Abnormal’s Respiratory Acidosis Respiratory Alkalosis Metabolic Acidosis Metabolic Alkalosis Respiratory Acidosis pH < 7.35 PaCO2 > 45 Alveolar Hypoventilation CNS depression or disease Ventilatory/respiratory dysfunction Acute infections Signs and Symptoms Dyspnea Restlessness Confusion/lethargy Dysrhythmias Headache Treatment Increase ventilation Treat the cause Respiratory Alkalosis pH > 7.45 PaCO2 < 35 Alveolar Hyperventilation Anxiety Pain Fever Thyrotoxicosis CNS lesions Improper vent management, hypoxia Pulmonary embolus Signs and Symptoms Light headedness Confusion Muscle spasm / parasthesias Dysrhythmias / palpitations Sweating Dry mouth Blurred vision Treatment Slow down breathing Paper bag breathing if anxiety or fear is cause Treat the cause! Metabolic Acidosis pH < 7.35 HCO3- < 22 mEq/L Causes Increased acids from Anaerobic metabolism Abn metabolic process (DKA, lactic acidosis) Starvation ASA or other acid ingestion Excess HCO3- loss Diarrhea Renal Failure Intestinal Fistulae Signs and Symptoms Headache Confusion/lethargy Stupor/coma Weakness Kussmaul respiration N/V Dysrhythmias Flushing Treatment Treat the cause!!! Give Bicarbonate Dialysis for renal failure Metabolic Alkalosis pH > 7.45 HCO3- > 28 Cause Increased base Excessive use of bicarb Lactate from dialysis Excessive antacid ingestion Acid loss Vomiting/Gastric suctioning Hypo- chloremia -kalemia Diuretics Large volume blood transfusion Signs and Symptoms Muscle spasms/tetany/seizure Dizziness Disorientation/lethargy/coma Weakness N/V Depressed respiratory drive Treatments Treat the cause!!!! Increase respirations Finished You have finished this in-service on Acid-Base Balance and Imbalance. Continue with the next lesson titled: Compensated and Uncompensated Blood Gas Analysis