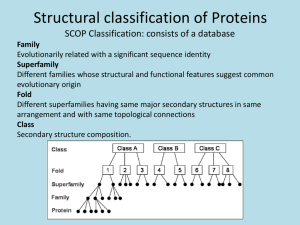
Protein Folding Pathways

Protein Toxicity in Parkinson
And Alzheimer’s Disease
Chuck Sanders, Dept. of Biochemistry
Rm 5110C MRBIII, chuck.sanders@vanderbilt.edu
Readings on Role of α-Synuclein in Parkinson Disease
100 years of Lewy pathology.
Goedert M, Spillantini MG, Del Tredici K, Braak H.
Nat Rev Neurol. 2013 Jan;9(1):13-24.
α-Synuclein: membrane interactions and toxicity in Parkinson's disease.
Auluck PK, Caraveo G, Lindquist S.
Annu Rev Cell Dev Biol. 2010;26:211-33.
Pathological roles of α-synuclein in neurological disorders.
Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L.
Lancet Neurol. 2011 Nov;10(11):1015-25
α-Synuclein oligomers and clinical implications for Parkinson disease.
Kalia LV, Kalia SK, McLean PJ, Lozano AM, Lang AE.
Ann Neurol. 2012 Aug 28. doi: 10.1002/ana.23746.
Misfolded protein aggregates: mechanisms, structures and potential for disease transmission.
(general, not α-synuclein specific)
Moreno-Gonzalez I, Soto C.
Semin Cell Dev Biol. 2011 Jul;22(5):482-7.
From: May 2010 US National Vital Statistics Report
PD
AD
Protein Deposition in Neurodegenerative Diseases: A Histological View
A. Abbott Nature Outlook 2011
NEJM 349, 583 (2003) note that list is incomplete and growing
Key Questions About Protein Aggregation in Alzheimer’s and Parkinson Diseases
What triggers protein aggregation?
In inherited form of disease?
In sporadic (idopathic) form of disease?
What form of the protein is toxic?
Disease promoted by toxic “gain of function”, loss of native function, or both?
What organ, tissue, and cells are affected… as a function of time?
Where in (or out) of cell does protein aggregate, deposit and exert its toxicity?
What is the mechanism of toxicity and is it direct or pathway-based?
Does protein deposition spread from cell to cell and tissue to tissue? If so, how?
Before we explore these issues, we need to review the biophysics and biochemistry of protein folding and misfolding.
Thermodynamic Stability of Proteins
unfolded folded
K eq,fold
C
K = eq,fold
[folded]
[unfolded]
G o
N
Standard Free Energies of Folding for Single Domain Proteins
Protein
G o fold
(kcal/mol) lambda repressor alpha spectrin SH3 domain
-3.0
-2.9
arc repressor -6.3
cytochrome C (with heme and Fe(II) -15
CD2 procarboxypeptidase
U1A spliceosomal protein
Hpr
-8.2
-4.1
-9.3
-4.6
Most folding proteins are only moderately stable:
The folded state is usually favored over the unfolded state by only -3 to -10 kcal/mol.
Many Proteins are Natively Unfolded (Natively Disordered)
The unfolded state is energetically favored, unless some the protein comes in contact with something that stabilizes the folded state (e.g., a cognate ligand or a membrane).
Polypeptides <50 Residues Usually Do Not Fold In Solution
Unless They Bind to Another Protein or to A Membrane
Protein Folding Pathways: Kinetics Revisited
G o
Thermodynamics provides the difference in energies between states and indicates whether “products” (i.e., folded state) or reactants (i.e., unfolded state) are favored. However, this tells nothing about the energetics of the pathway that is taken to get from one state to another. This is very important because the nature of the available pathway(s) will determine the rate of conversion between states. Indeed, if the pathways are too “difficult”, the process will occur at a negligible rate, no matter how favorable the
G o .
Just because of process is thermodynamically favorable, doesn’t mean the process will occur promptly – thankfully! Consider that peptide bond hydrolysis by water is extremely favorable from a thermodynamic standpoint… and yet peptide bonds spontaneously hydrolyze only very slowly.
Proteins fold because it is favorable from a thermodynamic perspective and also because there are kinetic pathways available that allow proteins to fold rapidly.
2 states (i.e., free ligand/protein vs. complex)
1 barrier in each direction
Kinetics
ΔG o gives the difference in energy between two states at equilibrium.
However, ΔG o does not directly indicate how fast the forward or reverse rates are. This is determined by the energy barriers ΔG * forward and ΔG * reverse
.
These "free energy of activation" determine the rate constants for the forward (k forwards
) and reverse (k reverse
) processes. The higher the barrer, the lower the rate constant, the lower the rate.
G o
G
* forward
G
* reverse
* rate is proportional to rate constant, k, which is proportional to:
e
G
RT
*
There is an inverse logrithmic relationship between rate and activation energy.
k = A .
e
G
RT
*
(the Arrhenius equation)
Rate is directly proportional to rate constant: k
G* ln k = + ln A (linear form of Ar. eq.)
RT
R is the gas constant, T is the absolute temperature, and A is a constant which is characteristic of a given process (but usually not interpreted.)
Mutations can perturb the heights of the rate barriers and/or the energies of the free energy states. Consider a mutation that lowers the energy of a folding intermediate:
unfolded
G
G o o
Examples of Potentially Deadly
Mutations
Barrier to folding becomes too high: protein folds too slowly and is degraded OR heads down an alternate pathway that leads to irreversible misfolding.
Normal barrier to misfolding is reduced so that alternate pathways leading to the misfolded form become efficient.
Mutation that lowers energy of folding intermediate (only).
Note that this mutation not only makes the intermediate much more stable (longer-lived), but that the energy barriers from this state to both initial and final states are now larger than for wild type.
folded
Folding intermediate is stabilized, giving intermediate time to irreversibly aggregate before folding is completed.
Folded form is destabilized, shifting equilibrium towards unfolded form, increasing the time spent in that aggregation-vulnerable form.
Interactions of a nascent protein with the cellular protein folding machinery is perturbed, so that folding assistance normally received by the protein is absent or corrupted to a bad end.
Protein Aggregation
When protein sticks to protein to form very large oligomers
Both folded and unfolded proteins can sometimes aggregate.
Aggregates can be disordered (amorphous) or can be ordered.
Amyloid fibrils are a common type of ordered aggregate.
The rate of formation of ordered aggregates is usually limited by the rate of a requisite nucleation step.
The Formation of Ordered Aggregates Is Rate-Limited By A Nucleation Event
This could involve the rare collision of minor conformations of the protein or could depend on the appearance of post-translationally modified form of a protein that can serve as an aggregation seed. The rareness of the seeding species and events can make it very hard to pin down the exact nature of the nucleation event, especially in living tissue.
Selkoe, Nature, 2003 cross-
β fibril formation on the road to mature amyloid; initial self-association might be staged from native or denatured states in some instances.
Protein Folding in the Cell
The main locations of protein folding in the cell are the cytosol and the lumen of endoplasmic reticulum.
ER: Membrane proteins, secreted proteins, and organellar proteins.
Protein folding in the cell…
While folded structure is determined by primary sequence and thermodynamics (as under test tube conditions), folding pathways may be different in cell than in studies involving purified protein, for the following reasons:
Folding is often co-translational. This means that the N-terminus starts folding as it comes out of the ribosome, even as the C-terminus is still being synthesized. This is especially important for multi-domain proteins because it allows domains to fold in an orderly sequential (one by one) fashion.
Chaperone proteins often play important roles in assisting folding and suppressing misfolding/aggregation in the cell.
Besides chaperones, there are a variety of other folding accessory proteins.
Folding is monitored by protein folding quality control.
Protein that is judged to be hopelessly defective by quality control is degraded (hopefully!)
There are two major degradation protein pathways in mammalian cells.
The ubiquitination/proteasome pathway.
The autophagy/lysosomal pathway(s).
Members of Alois Alzheimer’s research group at the Royal Psychiatric Clinic of the University of Munich, Germany in 1910
Alois
Alzheimer
Fritz Jakob
Heinrich Lewey
Goedert, M. et al
Nat Rev Neurol 9, 13-24, 2013
http://coloradodementia.org
Marwan Sabbagh
Extracellular Amyloid Plaques Are One of the Hallmarks of AD;
Central Component: The Amyloidβ Polypeptide
A Second Hallmark of Alzheimer’s Disease Are Intracellular Deposits Called
“Neurofibrillary Tangles”.
The Central Component of NFTs is the Hyperphosphorylated Tau Protiein
Intracellular Lewey Bodies Are the Hallmark Parkinson Disease
Central Component: α-Synuclein
The superstructure of amyloidβ in
(i) amyloid fibrils in amyloid plaques
(ii) hyperphosphorylated tau in neurofibrillar tangles,
(iii) α-synuclein in Lewey bodies is thought to be organized around
“amyloid” cross-beta fibrils formed by each of these three proteins.
The Amyloidogenic Pathway and Alzheimer’s Disease
cytosol
Amyloid Precursor
Protein
secretase cleavage
Amyloid-Beta cytosol
C99
secretase cleavage
Oligomers
Neuron
Cell Death
Nat Neurosci.
2012 Jan 29;15(3):349-57.
The toxic A
β oligomer and Alzheimer's disease: an emperor in need of clothes.
Benilova I , Karran E , De Strooper B .
The Amyloid Precursor Proteins and
The Amyloidβ Polypeptides
Long forms A β
42 and A β
43 thought to be more toxic than short forms A β
38 and A β
40
Retaining half of the TM segment of the APP, the A β polypeptides (especially) the long forms are only marginally water-soluble. They have a high propensity to aggregate and may also retain significant affinity for membrane surfaces.
The function of APP and of its fragments (extracellular domain: β-APP,
A β, and APP intracellular domain:AICD) are not well understood. Lots of bits and pieces of evidence in support of various roles. However, APP-knockout mice are a little slow and a little stupid, but live. Lethality occurs only when two homologs of APP – the APP-like proteins (ALP1 and ALP2) are also knocked out.
This implies that AD is not strongly related to the loss of native function of APP or of its derived fragments.
Formed extracellularly. However, some A β does seem to be found inside the cell in both lumenal compartments (esp. endosomes) and in the cytosol.
Schematic of the protein sequence of human α-synuclein.
Natively disordered in solution.
Binds to membrane surface via N-terminal domain, which is thought to form helices. C-terminal domain remains disordered, but engages in complexes with other proteins.
Found in cytosol, but some does seem to be able to escape from cells, pershaps via exosomes. Also can be found in the lumen of ER, endosomes, etc.
Bosco D A et al. Cold Spring Harb Perspect Biol
2011;3:a007500
©2011 by Cold Spring Harbor Laboratory Press
Functions of α-Synuclein
α-synuclein stabilizes synaptic vesicles (and other vesicles such as the ER-to-Golgi transport vesicles) to prevent premature membrane fusion and loss of vesicle contents. However, knockout of α-synclein is not fatal and does not lead to neurodegeneration. Parkinson’s does not seem to be primarily loss of function in nature.