Chapter 6, part I - Pleasant Hill R
advertisement
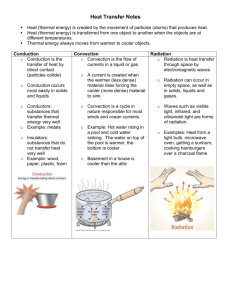
Physical Science Ch. 6: Thermal Energy Part I Heat, Temperature, and Thermal Energy • The Kinetic Theory of Matter states that all matter is made up of tiny particles (atoms or molecules) that are in constant motion (either vibrating in place, or actually moving around). • When materials are heated up, these particles move faster, and when materials cool, the particle speed decreases. • Temperature is a measure of the average kinetic energy of the particles in a sample of matter. • So what is the relationship between temperature and particle speed? • The SI unit for temperature is the Kelvin. Although the Celsius and Fahrenheit scales are more commonly used. Absolute Zero • As the temperature of a material decreases, particle movement slows. If the temperature decreases to a point where particle movement stops completely, this is called absolute zero. (0 Kelvin) • Absolute zero is theoretical, in that it has never been achieved. Thermal Expansion • The volume of an object will increase as it's temperature increases, and vice versa (directly proportional) Examples of thermal expansion: Shrink Fitting Heat • Heat is energy transfer from an object at a higher temperature to an object at a lower temperature. • Heat flow from a warmer object to a cooler object will continue as long as there is a temperature difference between the objects. • Once the temperatures of the objects are the same, they are said to be in thermal equilibrium. Les was camping in Colorado and ran out of water. So he took some water straight out of a stream, but decided to boil it first to kill any pathogens. The problem was, he only had a plastic 1 liter bottle to hold the water. Is it possible to boil the water in the plastic bottle? Explain. Explain why a bimetallic strip bends when it is heated. Energy Transfer • As we have already learned, energy transfer in the form of thermal energy will travel from an object or location at a higher temperature to one at a lower temperature. • There are 3 ways which this energy can be transferred. • Conduction is the transfer of energy through matter (usually solids, but not always) by direct contact between particles • For conduction to occur the materials must be: 1. In direct contact 2. At different temperatures • In conduction the heat is transferred from 1 particle to the next, as they vibrate. • Is it a very good idea to hold directly onto the handle of an iron skillet? • Why does heat always flow from a warmer object to a cooler object? • Different materials have different levels of conductivity. • Good conductors: metals • Poor conductors: wood, glass, plastic, rubber, cloth, air • Convection is the transfer of energy through the movement of matter. This occurs in fluids (liquids or gases). • Is convection the only way that a fluid can transfer energy? No! A hot water bottle, for example, gains heat from the warm water inside through conduction (direct contact with the warm water). So if the heat is being transferred through the motion of the fluid, then it is convection. If it is due just to direct contact, then it’s conduction. • Radiation is energy transfer through invisible waves which do not require matter. Examples: -sunlight -space heater -radiator -microwave oven • Tell whether each of the following is primarily an example of conduction, convection, or radiation. 1. ___An electric space heater in the corner of your room. 2. ___Having a VHS tape melt after being left in a hot car. 3. ___Car tires heating up as the car drives along. 4. ___Getting a sunburn. 5. ___Circulation of ocean currents. 6. ___The heat rising from a light bulb. 7. ___A warm breeze in your face. 8. ___Using a tanning bed. 9. ___Producing heat for your home with solar panels. 10. __Placing your hand in hot water. • On a hot August day, Bill wants to go fishing for muskie. So he calls his friend Roland who tells him, “Don’t you know that muskie won’t bite if the water temperature is over 80 degrees?” “Trust me” said Bill, “there’s a way around that”. What was Bill’s secret? Apu notices that some of the dairy products in his cooler at the Kwik-E-Mart are turning bad before the expiration date. He decides to check the temperature of his cooler to see if that is the problem. Does it matter where he places his thermometer in the cooler? Explain. • Flower baskets with balloons were given out to senior players on Senior Night. The gifts were purchased the day before and put in a cool area so that the flowers would not wilt. However, the next day the flowers looked fine, but the balloons were all half flat. Explain why this occurred and how this problem could be fixed. • Any material which is used to slow the flow of heat is called insulation. • Poor conductors make good insulators, and vice versa • R-value is a measure of the insulating ability of a material. The higher the r-value, the better an insulator a material is. • Why do you think some fiberglass insulation is backed with a thin layer of aluminum, if aluminum is a metal and therefore a poor insulator? • Thermography is the process of using special photographic film to show heat loss. • Thermography can be used to show heat loss from a house, and may indicate where more insulation or weatherproofing is needed. Specific Heat • As you may know, different materials heat up or cool down quicker than other materials. • For example, on the first warm day of the spring is it a good idea to go water skiing down at the lake? • The specific heat (Cp) of an object is the amount of energy needed to raise the temperature of 1 kg of a substance by 1 degree celsius. • So, the higher the specific heat of a material, does this mean that it will heat up faster or slower than an object with a lower specific heat? Examples: • Putting a pot full of water in an oven • Walking on a cold concrete floor, or a wooden floor • Burning your feet on the sand at the beach • Two horseshoes (iron and copper) are heated to the same high temperature and then plunged into separate buckets of 40 degree water and left there until thermal equilibrium is reached. • How could you predict which system will have the higher final temperature? Thermal Energy • Thermal energy is the total amount of energy of the particles in a sample of matter. • This is similar to the mechanical energy of an object, except this applies only to the particles. • Two identical tea kettles are heated until they start blowing steam and whistling. One was heated on a gas flame burner, while the other was heated on an electric burner. • Which one probably took longer to heat up, and which one probably cooled down the quickest after the heat was turned off? Why? • Bob leaves a pot of water outside in the shade all day, with a thermometer in it. He also leaves a thermometer sitting next to the pot. He checks both temperatures every 15 min. for 24 hrs. Will the temperatures always be the same, be different, or vary depending on the time of day? Explain. As Dwight and Jim were walking down the railroad tracks, Dwight noticed that the point where 2 pieces of track came together had about a ½” space between them. “Wow, they did a pretty sloppy job connecting these tracks”, Dwight commented. “They’re supposed to be like that, genius”, replied Jim. Explain Jim’s sarcastic response. • Larry was putting new steel siding on his house, and noticed that there were slots for the nails instead of round holes. He also heard that he needed to make sure not to hammer the nails in tightly, but to leave a slight gap between the nail head and the siding. Why? • Three beakers containing the same amount of water each have 2 drops of red food coloring dropped in. One beaker is at 30 degrees, one at 50, and one at 70. After 30 min. each beaker had pink water. If the water was still in each beaker, which one turned pink first, or did they turn at the same rate? Explain. • When choosing a cookware set, would it be best to choose one made of materials with a high specific heat or a low specific heat? Why? • Andy was running some errands on a warm summer day. He stopped by the grocery store, and then went to the library to do some reading. When he got home, he found that the rolls he had purchased were now busted open and had made a huge mess. Why did this happen? • Tell which of the following does not belong, and explain why: A. B. C. D. An egg in a microwave An aerosol can in a fire Canned food suck the lid down tight Running hot water over a jar lid Material Plastic Shot Pipe Insulation Wood chips Water Metal shot Air Change in Temp.