How To write Lewis structures
advertisement

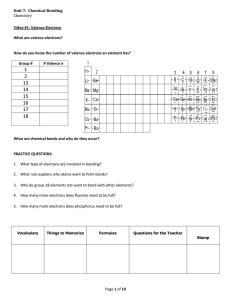
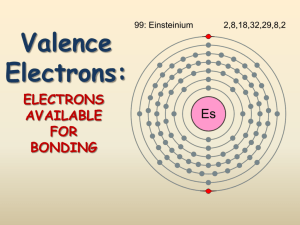
How To Write Lewis Structures 1. Draw skeletal structure a. H is always terminal b. Atom with the lowest E.N. (lowest IE) is central 2. Determine number of valence electrons 3. Determine number of electrons to get full valence shells 4. Bonding electrons = full valence shell – valence electrons 5. Draw bonding electrons in as single bonds 6. Draw remaining bonding electrons onto skeleton 7. If any valence electrons are left, draw them as lone pairs 8. Check formal charge Example: Draw Lewis Structure for Hydrogen Cyanide (HCN) and Cyanide Ion (CN-) How to Write Formal Charges (Not Oxidation Numbers) Formal Charge is the extent to which a single atom in a molecule has gained or lost and electron. FC = V – L – ½ S For an electrically neutral compound, sum of F.C. must be zero. For an ion, sum of FC must equal the charge on the ion. Expect more EN atom to have negative FC Examples: Write Lewis structures for Thionyl Chloride (SOCl2), Thiocyanate ion (SCN-) ion, CH3NHO- and Ozone (O3)