Session 1, Unit 1 Course Overview
advertisement

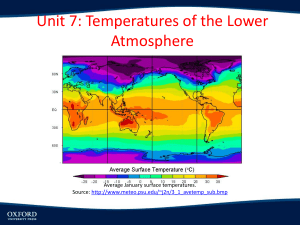
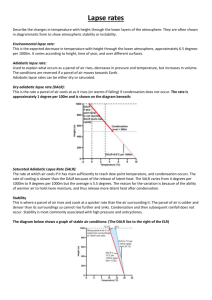
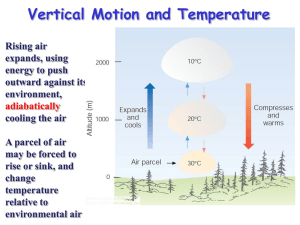
Session 2, Unit 3 Atmospheric Thermodynamics Ideal Gas Law Various forms m PV nRT RT M m PM V RT R P T M 1 Where Hydrostatic Equation Air density change with atmospheric pressure dP gdz dP g dz g dz dP First Law of Thermodynamics For a body of unit mass dq dw du dq=Differential increment of heat added to the body dw=Differential element of work done by the body du=Differential increase in internal energy of the body dw P d dq du P d cv dT P d Heat Capacity At constant volume dq du cv dT const dT const For ideal gas cv du dT At constant pressure dq cp dT p const Heat Capacity Relationships cv M C v cpM Cp C p Cv R Cp Cv Concept of an Air Parcel An air parcel of infinitesimal dimensions that is assumed to be Thermally insulated – adiabatic Same pressure as the environmental air at the same level – in hydrostatic equilibrium Moving slowly – kinetic energy is a negligible fraction of its total energy Adiabatic Process Reversible adiabatic process of air dq cv dT P d Add and subtract dP : dq cv dT d ( P ) dP Use ideal gas law : dq (cv R) dT dP dq c p dT dP Adiabatic process dq 0 c p dT dP 0 Combine with ideal gas law dP C p dT P R T Lapse Rate Combine hydrostatic equation and ideal gas law dP PM g g dz RT dP gM dz P RT For adiabatic process dP C p dT P R T Lapse Rate Therefore gM dT dz Cp dT/dz is Dry Adiabatic Lapse Rate (DALR) Dry Adiabatic Lapse Rate Dry adiabatic lapse rate (DALR) dT gM 9.81m / s 2 29 g / mol kg Pa m s 2 3 dz Cp kg 3.5 8.314m Pa / mol K 1000 g o o o K C F C 0.00978 9.78 5.37 10 m km 1000 ft km Or on a unit mass basis dT g 9.81m / s 2 9.8K / km dz c p 1004 J / kg K Or the expression in the textbook: dT ( g / g c ) 1 9.95 o C DALR dz R km Lapse Rate Effect of moisture C (1 w)C p, Air wC p,WaterVapor ' p Because C p (1 )C pAir CPWaterVapor C p ,W aterVapor C p , Air C p' C p Wet adiabatic lapse rate < DALR (temperature decreases slower as air parcel rises) Condensation Lapse Rate Superadiabatic lapse rate (e.g., 12oC/km) Subadiabatic lapse rate (e.g., 8oC/km) Atmospheric lapse rate Factors that change atmospheric temperature profile Standard atmosphere (lapse rate ~ 6.49 oC/km or 3.56 oF/1000 ft) Potential Temperature Current state: T, P Adiabatically change to: To, Po Po To T P 1 Set Po = 1000 mb, To is potential temperature If an air parcel is subject to only adiabatic transformation, remains constant Potential temperature gradient dT DALR z dz actual Session 2, Unit 4 Turbulence and Mixing Air Pollution Climatology Atmospheric Turbulence Turbulent flows – irregular, random, and cannot be accurately predicted Eddies (or swirls) – Macroscopic random fluctuations from the “average” flow Thermal eddies Convection Mechanical eddies Shear forces produced when air moves across a rough surface Lapse Rate and Stability Neutral Stable Unstable Richardson Number and Stability Stability parameter g s T z Richardson number Stable Neutral Unstable g z Ri _ 2 du T dz Stability Classification Schemes Pasquill-Gifford Stability Classification Determined based on Surface wind Insolation Six classes: A through F Turner’s Stability Classification Determined based on Wind speed Net radiation index Seven classes Feasible to computerize Inversions Definition Types Radiation inversion Evaporation inversion Advection inversion Frontal inversion Subsidence inversion Fumigation Planetary Boundary Layer Turbulent layer created by a drag on atmosphere by the earth’s surface Also referred to as mixing height Inversion may determine mixing height Planetary Boundary Layer Neutral conditions Mixing height u* h f Increased wind speed and surface roughness cause higher h. Planetary Boundary Layer Unstable conditions Mixing height t0 2 H dt t h dT C p DALR dz 1 2 Planetary Boundary Layer Stable conditions Mixing height u* h 0 .4 L f Surface Layer Fluxes of momentum, heat, and moisture remain constant About lower 10% of mixing layer Surface Layer Monin-Obukhov length L C p Tu *3 kgH Monin-Obukhov length and stability classes Surface Layer Wind Structure Neutral air u* z u ln ka z0 Surface Layer Wind Structure Unstable and stable air z z ln m L z0 For unstable air u u * ka 1 x 2 1 x m 2 ln ln 2arc tan( x) 2 2 2 z x 1 16 L For stable air z m 5 L 1 4 Friction Velocity ka u u* z z ln mk u z 0 u ln z L z a * z 0 m L Measurements of wind speed at multiple levels can be used to determine both u* and z0 Power Law for Wind Profile Wind profile power law u z u m zm Value of p p Estimation of Monin-Obukhov Length For unstable air For stable air z Ri L z Ri L 1 5 Ri Bulk Richardson Number dT DALR gz dz Rb 2 T u Rb Ri 2 p 2 Air Pollution Climatology Meteorology vs. climatology Meteorological measurements and surveys Pollution potential -low level inversion frequency in US Air Pollution Climatology Mean maximum mixing height determined by Morning temperature sounding Maximum daytime temperature DALR Stability wind rose