Integrated Rate Law
advertisement
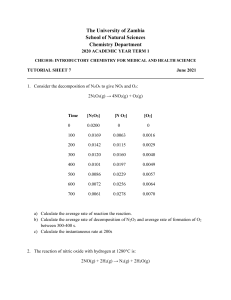
Rate laws can be converted into equations that tell us what the concentration of the reactants or products are at any time Calculus required to derive the equations but not to use them Rate = k With calculus it can be changed to an equation that relates the starting conc ([A]o) to the conc at any other time (t) [A] = -kt + [A]o Plot of [A] vs t is linear with a slope equal to k Find the conc of a reactant at some time after the reaction started Find the time required for a given fraction of a sample to react Find the time required for a reactant to reach a certain concentration Rate = k[A] ln[A] = - kt + ln[A]o Plot of ln[A] vs t is linear The first order constant for the hydrolysis of a certain insecticide in water at 12°C is 1.45/yr. A quantity of this insecticide is washed into a lake in June, leading to an overall concentration of 5 x 10-7 g/cm3 of water. Assume that the effective temp of the lake is also 12°C. A) what is the conc of the insecticide in June of the following year? B) How long will it take for the conc of the insecticide to drop to 3.0 x 10-7 g/cm3 ? The decomposition of dinitrogen pentoxide is studied over time and the results are given in in the table on pg.573. a) Verify if this is a first order reaction. b) Calculate the value of the rate constant c) Find the concentration after 150 sec. Time required for the concentration of a reactant to decrease to halfway between its initial and final values Time when [A]= ½ [A]o t1/2 = .693/k What is the half life of the insecticide in the lake from the previous example? Rate = k[A]2 1/[A] = kt + 1/[A]o Plot of 1/[A] vs t is linear Plot of 1/[A] vs t is linear The following data was obtained for the decomposition of nitrogen dioxide. Is the reaction first or second order? What is the rate constant? Time (s) [NO2] 0 .0100 50 .0079 100 .0065 200 .0048 300 .0038