Primary Care and Chronic Pain management
advertisement

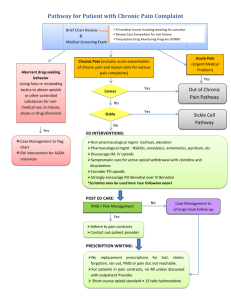
PRIMARY CARE AND CHRONIC PAIN MANAGEMENT Kristin L. Kuhlmann, Ph.D., APRN, FNP-BC West Texas A & M University Assistant Professor, Graduate Nursing Program Director, WTAMU Health Partner’s Clinic 22nd Annual Panhandle Nurse Practitioner Association Symposium Amarillo, TX April 11, 2015 Objectives 1. Identify physiological differences between acute and chronic, non-malignant pain. 2. Describe the important pharmacodynamic and pharmacokinetic differences among opioids, and adjuvant pain medications, with rationale for the pharmacotherapeutic plan. 3. Describe other modalities for chronic pain management. 4. Discuss important inter-professional clinical considerations related to chronic pain management. 5. Discuss the importance of a “healthy” patient/provider relationship for effective pain management. *No financial/non-financial conflicts of interest* Controlled Substances Updates Texas Board of Nursing • In addition to the 20 contact hours of continuing nursing education in the advanced practice role and population focus area or renewal of the certification, an APRN who has Prescriptive Authority must also complete a minimum of 5 additional contact hours in pharmacotherapeutics within the preceding 2 years. Texas Nurse Practitioners Association • Controlled Substances CE • A total of 3 hours is required for APRNs with prescriptive authority who order/ prescribe controlled substances for every licensure cycle after January 1, 2015. Those who renew this year do not need this CE, but will need it in the next licensure cycle. Reclassification of Medications by DEA • August 18, 2014- Tramadol classified as Schedule IV • October 6, 2014- Hydrocodone combination products (HCPs) reclassified as Schedule II *Only APRNs working in hospital-based practice or hospice-affiliated facilities can prescribe Schedule II medications in Texas.* Controlled Substances • The U.S., Drug Enforcement Agency (DEA) have delineated five categories, or schedules for controlled drugs: • Based on accepted medical use • Potential for dependency and abuse • Health care providers are provided with both a DEA and state license to prescribe, distribute, or administer controlled substances. • General Classes/Uses: • Pain Management • Anxiety • Sleep Disorders • ADHD DEA Controlled Substance Schedules Schedule I No consensus- accepted medical use High potential for abuse Severe psychological or physical dependence heroin, LSD, marijuana, methaqualone, peyote Schedule II High potential for abuse Potentially severe psychological or physical dependence Considered “dangerous” Schedule III Moderate to low potential for psychological/ physical dependence Drug abuse potential lower than Schedule II Schedule IV Low risk for potential abuse and dependence Schedule V Lower potential for abuse or dependence Opioids: hydrocodone, morphine, methadone, hydromorphone, meperidine, oxycodone, fentanyl Amphetamines: methamphetamines, cocaine, dexedrine, Adderall, Ritalin Opioids: codeine Anesthetic: Ketamine Male sex hormones: anabolic steroids, testosterone Opioids: Tramadol, Talwin, Talwin Nx Benzodiazepines: Xanax, Ativan, Soma, Valium Sedative: Ambien(zolpidem) Anticonvulsant/neuropathy: Lyrica (pregabalin) Cough preparations: Less than 200 mg of codeine Antidiarrheals: Lomotil, parapectolin, Motofen All scheduled drugs/substances have potential for dependence and abuse. Schedule I substances are the most “dangerous”; Schedule V have the lowest potential for dependence and abuse. Opioid Agents/ Narcotics OPIOID AGENTS (GENERIC NAMES) OPIOID AGONISTS PHENANTHRENES- SCHEDULE II morphine sulfate (extended release) morphine sulfate (short acting) hydrocodone with acetaminophen hydrocodone with ibuprofen hydrocodone high dose oxycodone oxycodone with aspirin oxycodone with acetaminophen hydromorphone codiene with acetaminophen- SCHEDULE III PHENYLPIPERIDINES- SCHEDULE II meperidine fentanyl DIPHENYLHEPTANES- SCHEDULE II methadone MU RECEPTOR AGONIST & NE REUPTAKE INHIBITOR- SCHEDULE II tapentadol, tapentadol ER MIXED AGONIST/ANTAGONIST- SCHEDULE IV pentazocine; pentzocine with nalaxone PARTIAL AGONISTS- SCHEDULE IV Tramadol; tramadol with acetaminophen BRAND NAMES MS Contin, Avinza, Kadian, Embeda, Oramorph SR, generic MS-IR, Kadian, MS Lortab, Vicodin, Norco Vicoprofen Zohydro ER Oxycontin, Oxy IR, Oxecta Percodan Endocet, Percocet, Roxicet, Tylox Dilaudid, Exalgo ER Tylenol #3, Tylenol #4 Demerol Duragesic transdermal, Actiq (transmucosal), Abstral, Fentora, Onsolis, Lazanda Dolophine Nucynta; Nucynta ER Talwin; Talwin Nx Ultram; Ultracet Neurobiology of Narcotic Use • Like food and sex, opioids (narcotics) stimulate mu receptors of the mesolimbic reward system in the midbrain: • Generate signals to cells in the Ventricle tegmental area (VTA) • Releases the neurotransmitter, dopamine, into the Nucleus accumbens • Elicits feelings of pleasure/euphoria with pain relief • This reward circuit includes areas of motivation and memory, encouraging repeated use or behavior. • The Hippocampus stores memories of rapid satisfaction induced by the drug. • With repeated use, the Prefrontal cortex, with normal functions of planning and executing responsible actions, becomes involved in craving activities: • Override inhibitory signals in some individuals, overuse and addictive behaviors begin. Hippocampus Tolerance • Narcotics provide a shortcut to endorphin release • Quickly flood the brain with dopamine and other neurotransmitters, at 2-10 times the amount of natural induction. • With repeated use of narcotics, ventricle tegmental area (VTA) receptors in the midbrain get overwhelmed • Seeking homeostasis, the VTA: • Produces less dopamine and some dopamine receptors are inactivated • An increase in drug dosage is needed for the same effect over time (tolerance) • Repeated exposure to escalating doses of the narcotics alters brain function: • Functions “normally” when drug present, and abnormally when absent • Drug dependence occurs • Without the drug, withdrawal symptoms occur Narcotic Withdrawal, Overuse, Addiction Orexin activates enzymes that convert ATP into another chemical, cAMP: • Triggers the release of norepinephrine, stimulating normal functioning, such as wakefulness, appetite, muscle tone, general feeling of well being. When a narcotic links to the mu opioid receptors: • Enzymes are inhibited, less cAMP is produced, • Less norepinephine is released: • Opioid effects of sedation, shallow breathing, incoordination, slurred speech occur With repeated opioid use, neurons produce more cAMP to offset the inhibitory effect of drug: • Clinical symptoms of withdrawal symptoms occur: • Jitters, anxiety, muscle cramps, insomnia, dilated pupils, diarrhea, nausea and vomiting. • These unpleasant symptoms play a part in the cycle of overuse, physical and psychological addiction. Narcotic Overdose Deaths • In 2011, there were 1.5 million emergency room visits for pharmaceutical overdoses. • Annually, drug overdoses kill more people than motor vehicle accidents. • The drug overdose death rate has doubled since 1999, largely due to the increased use of narcotics in the outpatient population. Overdose Deaths • In 2013, there were 23,000 deaths from pharmaceutical overdoses. • Every day in the U.S., 120 people die and 6,750 people are treated for drug overdose. • Of pharmaceutical overdose deaths, 70% result from opioids and 30% from benzodiazepines. • 81% of pharmaceutical overdose deaths are unintentional. Pain- Definitions and Categories • Definition of pain: • Unpleasant sensory and emotional experience • Bodily sensation of tissue damage occurring • An experience of a threat is associated with the sensation, prompting action to make it stop • Two categories of pain: • Adaptive (acute)- necessary for survival, protection from further injury, body response to promote healing • Maladaptive (chronic)- part of a disease process; pathologic functioning of the nervous system (neuropathy) • Lasts beyond the time necessary for injury of the body to heal • Duration of 3 to 6 months, or longer Transmission of Pain • Transduction- conversion of thermal, mechanical, or chemical electrical activity at peripheral sensory nerve endings (nocioceptors) • Transmission- through laminae of dorsal horn of the spinal cord to the brain stem, and through connections between the thalamus and brain cortex • A-delta nerve fibers are myelinated, quick transmission of pain, intense sensation • C-fibers are non-myelinated; delayed, throbbing, dull, longer-lasting pain; emotional response elicited • Modulation- In the spinal cord, the sensation is augmented by excitatory neurotransmitters (ascending) and sent up to the brain; • Brain sends down inhibitory neuropeptides (descending) • Perception- Interpretation of input in brain gives rise to specific sensory consciousness, a multidimensional experience of pain Perception of Pain Pain stimulates multiple areas of the brain; response is elicited by three major areas: 1. Somatosensory cortex • 3-neural relay system • From peripheral sensory nocioceptors to dorsal root ganglia (first order neuron cell bodies) • Within spinal cord (second order neuron cell bodies) • Sensation of pain, touch, temperature relayed to: • Medulla evaluates stimuli- touch, vibratory sense, position • Through brainstem to thalamus nucleus (third order neuron cell bodies) • Impulse then sent to anterior cingulate cortex: • Intensity, type, and location of pain interpreted and analyzed • Sensation related to memory of past experiences; formulate cognitive assessment of level/ management of painful stimuli Perception of Pain 2. Reticular activating system (RAS)- Located in brainstem • Part of the mammalian brain, responsible for sleep, waking, elimination • Drive for sex, eating, breathing, and heart rate • Ascending neural fibers connect with hypothalamus, thalamus, and cortex • Descending neural fibers connect with cerebellum and sensory nerves • Motor response to pain • e. g.: Moving the hand quickly away from a hot surface • Affective-motivation response • Assess the level of injury; limits or restriction of movement 3. Limbic system (Reward)- Thalamus • Emotional and behavioral responses to pain • Affects mood, responsible for perception and the motivation to respond to the pain experience Neural Pain Pathway Adapted from: A. K. Srivastava. (2010). Pain: Physiological considerations. Retrieved from HomeOrizon website: http://www.homeorizon.com/homeopathic-articles/neurology/painphysiological-consideration Pain Receptor Sites and Opioid Action Receptor Mu1 Mu2 Kappa1 Kappa2 Kappa3 Delta Location/Function Supraspinal, periphery Analgesia Spinal analgesia Spinal, mediates visceral pain Spinal, mediates visceral pain Supraspinal analgesia Supraspinal, Spinal antagonist activity only, no delta agonists developed yet Side Effects Euphoria, respiratory depression Opioids Morphine-like narcotics Respiratory depression, physical dependence, (Table 1), decreased GI motility, pruritus endorphin neuropeptides Spinal analgesia; sedation, miosis See Table 1 below, Dysphoria morphine(slight), Dynorphin neuropeptides Antidepressant effect, convulsant effect, Antagonists of delta receptortachycardia, tachypnea, hallucinations, buprenorphine, trazodone mydriasis, hypertonia Enkephalin neuropeptides Types of Pain • Nocioceptive pain- pain along a nerve fiber, usually due to tissue damage • Somatic- caused by injury to body tissues • Well localized, but variable in description and experience • Musculoskeletal (fractures, sprain/strains) • Inflammatory (arthritis, bursitis, infection, gout) • Mechanical/compressive (low back pain, neck pain, crush injury) • Visceral- caused by stretch receptors within or surrounding the chest and abdominal (internal) organs • Poorly localized- cramping, shooting, stabbing, aching, burning • Inflammatory- (pneumonia, appendicitis, urinary tract infection) • Mechanical/compressive -(tumors, growths, scarring, shifting/prolapse) Treatment of Nocioceptive Pain Identified Risks and Treatment Considerations Chronic kidney disease, advanced age - avoid NSAIDs and COX-2 inhibitors Peptic ulcer disease, glucocorticoid use - avoid NSAIDs Hepatic disease - avoid NSAIDs, COX-2 inhibitors, and acetaminophen (APAP); use TCAs or duloxetine first line Cardiovascular disease or risk - use lowest effective dose of NSAIDs; in patients who require treatment, suggest naproxen Adapted from: T. M. Woo, & A. L. Wynne, (Eds.). (2012). Pharmacotherapeutics for nurse practitioner prescribers (3rd ed.). Philadelphia, PA: F.A. Davis Company. NSAID: nonsteroidal anti-inflammatory drug; COX-2 inhibitor: cyclooxygenase 2 inhibitor; APAP: acetaminophen/paracetamol; TCA: tricyclic antidepressant; PPI: proton pump inhibitor. Types of Pain (cont.) • Neuropathic- Abnormal neural activity due to disease, injury, or dysfunction of the nervous system • Sympathetically mediated pain (SMP)- complex regional pain (reflex sympathetic dystrophy)- post-injury, triggering of immune response • Peripheral neuropathic pain – post herpetic neuralgia, neuroma • Central nervous system pain- phantom limb pain, spinal cord injury, post-stroke pain • Classification of Neuropathy • Mononeuropathy- one nerve affected (e.g. carpal tunnel syndrome) • Mononeuropathy multiplex- several nerves affected, multifocal • Numbness, tingling, abnormal sensation (e.g., diabetic neuropathy) • Polyneuropathy- symptoms are diffuse and bilateral (e.g., fibromyalgia; stocking/glove neuropathy) Treatment of Neuropathic Pain SNRI: serotonin-norepinephrine reuptake inhibitor; TCA: tricyclic antidepressant; NMDA: N-methyl-D-aspartate. Adapted from: T. M. Woo, & A. L. Wynne, (Eds.). (2012). Pharmacotherapeutics for nurse practitioner prescribers (3rd ed.). Philadelphia, PA: F.A. Davis Company. Acute and Chronic Pain • Acute Pain • Sudden onset • Usually from a clearly identifiable cause • Treatment is designed to prevent further injury and treat pain • Pain totally resolves with healing of the injury • Chronic Pain • Persists for weeks to months • Often associated with an underlying medical condition • Treatment goal is to return the patient to optimal function • Total eradication of pain may not be possible • Treatment • Pharmaceutical/Medications • Other Treatment Modalities • • • • • Physical therapies Psychological support Multiprofessional health support Family/Social support Lifestyle modifications Non-Pharmacologic Measures • Physical measures • Heat, ice, massage, splinting/support, manipulative therapies (e.g. chiropractic, osteopathy), physiotherapy • Treat primary cause • Improve diabetic control, reduce weight if overweight/obese; moderate, regular exercise; reduce/stop alcohol consumption, vitamin/mineral supplementation (thiamine, niacin, Vitamin D, copper, selenium, magnesium). • Surgical interventions to correct defect structural defects (e.g.: disc prolapse, spinal stenosis, carpal tunnel syndrome) • Inhibitory stimulation of the periphery/spinal cord • Acupuncture/TENS • Electrical peripheral nerve or dorsal column stimulation • Central (deep-brain) stimulation • Inhibition or prevention of ascending nerve transmission from periphery/ spinal cord • Nerve blocks • Neurolysis or rhyzolysis • Alter pain processing at the cortical level • Cognitive therapies, biofeedback, hypnosis, meditation • Improved effect on descending inhibition • Decreased sensitivity to ascending painful stimuli Acute Pain Management Algorithm Chronic Pain • Over 100 million Americans suffer chronic pain annually • 1 in 3 people will experience chronic pain at some time • 80-90% experience pain in their neck or lower back • 20% of outpatient visits and 12% of all prescriptions written in the U.S. are for chronic pain • In 2010, 19% of adults reported constant or frequent pain, with most stating pain is moderate or severe • Cost of untreated/undertreated pain- $100B annually • Overall, 35% of patients with chronic pain have PTSD • PTSD-related neurohormones, neurotransmitters, and inflammatory system factors transmit and/or amplify pain stimuli: • 98% of persons with PTSD will experience chronic pain in their lifetime Chronic Pain Treatment Algorithm Copyright 2012 F.A. Davis Company www.fadavis.com Establishing/Maintaining a Healthy Relationship: Chronic Pain Management • Detailed history and physical to assess the pain condition (biopsychosocial approach) • Identify the specific context of how the patient’s pain interferes with ADLs, including sleep, work, social events (family/friends/church), emotions, coping, side effects of treatment • Obtain records from pain management provider • Collaborate to provide best care for patient • Mutual establishment and understanding of realistic treatment goals and expectations • Pain may not be fully alleviated, but improvement in ADLs is goal • Acknowledge and validate the patient’s pain symptoms • Formulation of a meaningful treatment plan • Input of patient’s specific needs • Be clear about comfort level/agreement about prescribing medications • Participatory decision making • Modification of the treatment plan • Re-assessment and communication with the patient on follow-up visits