this slide kit
advertisement
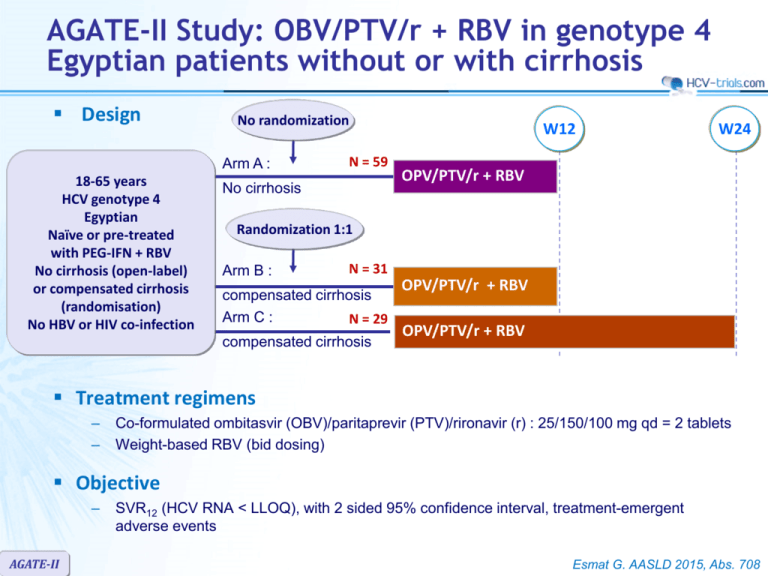
AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis Design No randomization Arm A : 18-65 years HCV genotype 4 Egyptian Naïve or pre-treated with PEG-IFN + RBV No cirrhosis (open-label) or compensated cirrhosis (randomisation) No HBV or HIV co-infection N = 59 No cirrhosis W12 W24 OPV/PTV/r + RBV Randomization 1:1 Arm B : N = 31 compensated cirrhosis Arm C : N = 29 compensated cirrhosis OPV/PTV/r + RBV OPV/PTV/r + RBV Treatment regimens – – Co-formulated ombitasvir (OBV)/paritaprevir (PTV)/rironavir (r) : 25/150/100 mg qd = 2 tablets Weight-based RBV (bid dosing) Objective – AGATE-II SVR12 (HCV RNA < LLOQ), with 2 sided 95% confidence interval, treatment-emergent adverse events Esmat G. AASLD 2015, Abs. 708 AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis Baseline characteristics No cirrhosis 12W N = 100 Cirrhosis 12W N = 31 Cirrhosis 24W N = 29 49 57 56 Female 30% 6% 24% Mean BMI, kg/m2 29.1 29.3 31.0 Mean HCV RNA, log10 IU/Ml 6.0 6.0 6.0 68 / 11 / 19 / 2 0 / 0 / 3 / 97 0 / 0 / 0 /100 Treatment-naïve, % 49 48 52 Treatment-experienced, % Null responder Partial responder Relapser 33 8 10 29 6 16 24 7 17 Mean platelet count (x 109/l) 229 156 138 44.7 ± 3.0 41.8 ± 4.4 40.3 ± 5.3 15 29 24 Mean age, years Fibrosis stage (%) : F0-F1 / F2 / F3 / F4 Mean albumin (g/l) History of diabetes, % AGATE-II Esmat G. AASLD 2015, Abs. 708 AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis SVR rates by treatment arm 100 94 97 97 80 60 40 20 28/29 % Data Pending – Study in Progress % SVR12 by baseline fibrosis stage (12 week treatment arms) 94/100 30/31 SVR12 SVR12 SVR4 SVR12 Arm A No-cirrhosis 12 weeks Arm B Cirrhosis 12 weeks Arm C Cirrhosis 24 weeks 0 AGATE-II 100 95 94 94/99 30/32 < F3 = F4 80 60 40 20 0 Baseline fibrosis stage Esmat G. AASLD 2015, Abs. 708 AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis Reasons for not achieving SVR12 Non-response On-treatment virologic failure Premature study drug discontinuation Missing SVR12 data Relapse by W12 post-treatment No cirrhosis W12 Cirrhosis W12 Cirrhosis W24 2/100 (2.0%) 1/31 (3.2%) 1/29 (3.4%) 1 (1.0%) * 1 (3.2%) 1/29 1 (1.0%) withdrew consent 0 0 1/100 0 NA** 3/98 (3.1%) * 0 NA** * Includes 1 patient with compensated cirrhosis miscategorized as non-cirrhotic and assigned in Arm A ** Study ongoing AGATE-II Esmat G. AASLD 2015, Abs. 708 AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis Treatment-emergence adverse events No cirrhosis W12 N = 100 Any treatment-emergent adverse event 72 (72%) Adverse event leading to discontinuation 0 Serious adverse event, % 2 Severe adverse event, % 2 Death, N 1 Adverse events occurring in ≥ 10% in either group, % Headache 40 Fatigue 33 Pruritus 23 Dyspepsia 15 Upper abdominal pain 16 ALT ≥ Grade 2 (> 3 x ULN) 0 AST ≥ Grade 2 (> 3 x ULN) 0 Total bilirubin grade 3 (> 3-10 x ULN), % 2 Hemogloblin 8-10 g/dl, N (%) 7 (7) RBV dose reduction, N 10 AGATE-II Cirrhosis W12 N = 31 Cirrhosis W24 N = 29 21 (68%) 0 0 0 0 25 (86%) 0 7 7 0 29 23 13 10 6 0 0 6 2 (6) 4 34 38 31 14 10 0 0 14 4 (14) 7 Esmat G. AASLD 2015, Abs. 708 AGATE-II Study: OBV/PTV/r + RBV in genotype 4 Egyptian patients without or with cirrhosis Summary – High SVR rates were achieved in HCV genotype 4-infected Egyptian patients without cirrhosis or with compensated cirrhosis after 12 weeks of OBV/PTV/r + RBV weeks: SVR12 was 94% and 97%, respectively – 12 weeks treatment was well tolerated with no treatment discontinuations due to adverse events – Prolongation of therapy to 24 weeks does not provide additional benefit, with more serious adverse events and a higher frequency of hemoglobin decrease AGATE-II Esmat G. AASLD 2015, Abs. 708