Reaction Types
advertisement

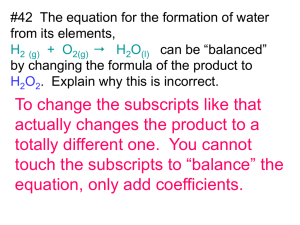
Reaction Types How do we know what will be produced in a chemical reaction? Recognizing a pattern of reactivity gives a broader understanding than memorizing a large number of reactions Would expect elements of the same family to behave similar 2K + 2H2O → 2KOH + H2 What will happen if Na combines with water? Five Types of Reactions Combustion – rapid reaction that produces flame Occurs in hydrocarbons (compounds of C, H, and sometimes O) Reaction of hydrocarbon with oxygen to produce carbon dioxide and water C3H8 + 5O2 → 3CO2 + 4H2O 2CH3OH + 3O2 → 2CO2 + 4H2O Combination or Synthesis Two reactants combine to form a single product A + B → AB Examples C + O2 → CO2 N2 + 3H2 → 2NH3 CaO + H2O → Ca(OH)2 Decomposition Single reactant breaks apart to form two or more substances AB → A + B Examples 2KClO3 → 2KCl + 3O2 CaCO3 → CaO + CO2 Single Replacement One element replaces another similar element in a compound A + BX → AX + B Examples Fe + 2HCl → FeCl2 + H2 Zn + CuSO4 → ZnSO4 + Cu Na + 2H2O → 2NaOH + H2 Double Replacement Atoms exchange partners Also called metathesis reactions AX + BY → AY + BX We will look at two types of double replacement Acid-base reactions Precipitation reactions Acid-Base Reactions Acid – substance that increases the H+ concentration in aqueous solutions HCl → H+ + ClH2SO4 → H+ + HSO4HSO4 → H+ + SO42- Base Increases the OH- concentration NaOH → Na + OHNH3 + H2O → NH4+ + OH- Neutralization Acids and bases react together to produce a salt (ionic compound) and water HCl + NaOH → NaCl + H2O Write a balanced equation for the reaction of hydrobromic acid, HBr, with barium hydroxide, Ba(OH)2. Write the balanced equation for the reaction between phosphoric acid, H3PO4, and potassium hydroxide. Precipitation Reaction Double replacement that forms a precipitate KI (aq) + Pb(NO3)2 (aq) → PbI2 (s) + 2KNO3 (aq) Example When sodium phosphate and barium nitrate are mixed, a precipitate of barium phosphate forms. Write the balanced equation. Example When aqueous sodium hydroxide and magnesium nitrate are mixed, a magnesium hydroxide precipitate forms. Write the balanced chemical equation.