Elements and periodic table
advertisement

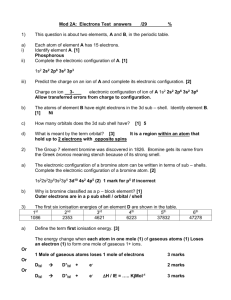
Learning Outcomes • Atomic radii (covalent radii only). Explanations for general trends in values: (i) down a group (ii) across a period (covalent radii of main group elements only). • First ionisation energies. • Explanations for general trends in values: (i) down a group (ii) across a period (main group elements) and for exceptions to the general trends across a period. • Second and successive ionisation energies. • Evidence for energy levels provided by successive ionisation energy values. Atomic radii Atomic radii trends • In general, the atomic radii values decrease across the period and increase down the group Atomic radius • Half the distance • Between the nuclei of 2 atoms of the same element • Joined by a single covalent bond trends Reasons for increase down a group • The additional electrons are going into a new shell which is further from the nucleus • Screening effect of inner electrons Screening effect Reasons for decrease across a period • Increasing nuclear charge. • No increase in the screening effect IONISATION ENERGY • Some elements lose electrons very easily, e.g. sodium and potassium • Silver and gold have very little tendency to lose their electrons and hence are very unreactive definition The first ionisation energy is the energy required to remove the most loosely held electron from one mole of gaseous atoms to produce 1 mole of gaseous ions each with a charge of 1+. Na loses an electron equation Na Na + + e I. E. in groups I and II Chlorine increases in size Ionisation Energy decreases going down a group Reasons for decrease down • Increasing atomic radius. • Screening effect of inner electrons Ionisation Energy increases across a period Reasons increase across • Increasing nuclear charge. • Decreasing atomic radius IE and periodic table. Exceptions to the general trends • Beryllium and nitrogen have higher values than expected • Reasons: • Be 1S2 2S2 (Full orbitals give greater stability) • N 1S2 2S2 2Sx1 2Py1 2Pz1 (3 half filled orbitals give greater stability) EVIDENCE FOR EXISTENCE OF ENERCY LEVELS • Suppose we measure the first, second, third, etc. up to the nineteenth ionisation energy of potassium • K = 1S2 2S2 2P6 3S2 3P6 4S1 • K = 2,8,8,1 • First ionisation energy has the lowest ionisation energy value. Electron in the 4s sublevel is easiest to remove Potassium ionisation • 1st ionisation energy: K(g) → K+(g) + e– • 2nd ionisation energy: K+(g) → K2+(g) + e– n=1 n=2 n Potassium IE’s ionisation energy (kJ mol–1) Sucessive Ionisation of Potassium 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 419 3051 4412 5877 7975 9649 11343 14942 16964 48577 54433 60701 68896 75950 83152 93403 99771 444911 476075 500000 450000 400000 350000 300000 250000 200000 150000 100000 50000 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Series1 Potassium IE’s Sucessive Ionisation of Potassium 500000 450000 400000 350000 300000 A 250000 200000 150000 B C 100000 50000 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Series1 14 15 16 17 18 19 • A, one electron has been removed from potassium • The second electron is much more difficult to remove since this electron is being removed from the K+ ion Potassium IE’s Sucessive Ionisation of Potassium 500000 450000 400000 350000 300000 250000 200000 150000 B C 100000 50000 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Series1 14 15 16 17 18 19 • K+ This ion has eight electrons in the outer shell ( B,C) • The full outer sublevel (3p6) has extra stability and therefore will require more energy to remove electrons from it. (B,C) Potassium IE’s Sucessive Ionisation of Potassium • B to C we are removing eight more electrons • (point C on the graph), there is another sudden jump D is being removed from a shell which is closer to the nucleus 500000 450000 400000 350000 300000 D 250000 200000 B C 150000 100000 50000 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Series1 14 15 16 17 18 19