Unit Ten Note Guide
advertisement
Unit Ten: Gases
Note Guide
1.
Name: __________________________
Hour: ______
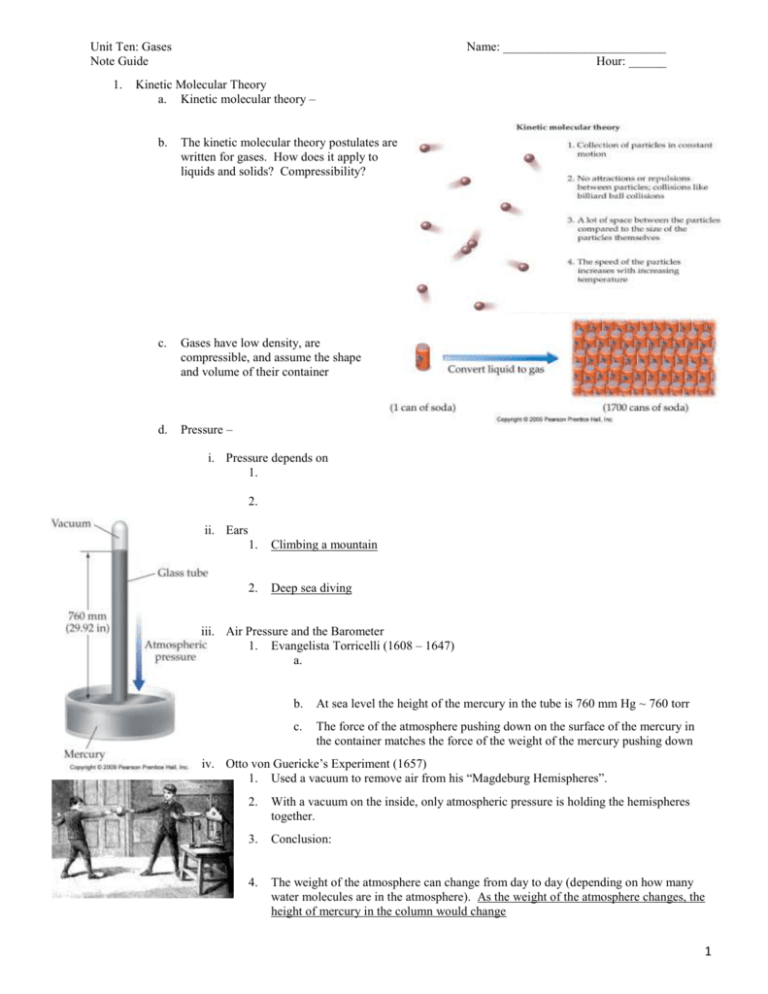
Kinetic Molecular Theory
a. Kinetic molecular theory –
b.
The kinetic molecular theory postulates are
written for gases. How does it apply to
liquids and solids? Compressibility?
c.
Gases have low density, are
compressible, and assume the shape
and volume of their container
d.
Pressure –
i. Pressure depends on
1.
2.
ii. Ears
1.
Climbing a mountain
2.
Deep sea diving
iii. Air Pressure and the Barometer
1. Evangelista Torricelli (1608 – 1647)
a.
b.
At sea level the height of the mercury in the tube is 760 mm Hg ~ 760 torr
c.
The force of the atmosphere pushing down on the surface of the mercury in
the container matches the force of the weight of the mercury pushing down
iv. Otto von Guericke’s Experiment (1657)
1. Used a vacuum to remove air from his “Magdeburg Hemispheres”.
2.
With a vacuum on the inside, only atmospheric pressure is holding the hemispheres
together.
3.
Conclusion:
4.
The weight of the atmosphere can change from day to day (depending on how many
water molecules are in the atmosphere). As the weight of the atmosphere changes, the
height of mercury in the column would change
1
v. Predicting the weather:
1. High Atmospheric Pressure = ______________________
2.
Low Atmospheric Pressure = ______________________
3.
Why? What molecules are present in the atmosphere?
vi. Units of Pressure
1. Standard pressure
a. 1 atm
b. 101.325 kPa
c. 101,325 Pa
d. 1.01325 bar
2.
e.
f.
g.
760 mm Hg
760 torr
14.7 psi (lb/in2)
Practice Questions
a. A liquid with twice the density of water is used in a barometer under normal
atmospheric pressure. What would the height of the barometer be?
b.
If the pressure on top of Mount Everest is 0.311 atm, what is the pressure in
mmHg?
c.
Airplanes that fly above 25,000ft must have pressurized cabins. According to
FAA regulations, cabin pressure must be at or above 0.72 atm (= air pressure
at 8,000 ft). Calculate the pressure in the cabin at 0.72 atm for the following:
i. mm Hg
ii. lbs/in2 (psi)
iii. kPa
iv. If the pressure in the airplanes is 500 mmHg, does this meet FAA
regulations?
2.
Ideal Gas Law
a. Ideal gas law is PV=nRT
i. P =
iv. R =
ii. V =
v. T =
iii. n =
b.
If pressure increase and T and n stay constant, what will happen to the volume?
c.
If temperature increases and P and n remain constant, what will happen to the volume?
d.
If the number of moles increases and P and T stays constant, what will happen to the volume?
e.
The temperature scale cannot go negative because it would result in a negative value for volume of
matter and we cannot divide by zero so we use the ___________________________________.
2
i. To convert between °C and K we use the equation: K = °C + 273 (or °C = K – 273)
1.
-107 °C = _________
4.
51 K
= _________
2.
27 °C
= _________
5.
354 K
= _________
3.
103 °C = _________
6.
101 K
= _________
ii. Experimental results show than an increase of 1°C results in a volume increase of _________ of
the original volume resulting in the new total volume of _________.
f.
1.
99 °C
= _________
3.
-1 °C
2.
1 °C
= _________
4.
-105 °C = _________
R is the ideal gas constant. It has units of
= _________
𝑝𝑟𝑒𝑠𝑠𝑢𝑟𝑒 ∙ 𝐿
𝑚𝑜𝑙𝑒𝑠 ∙ 𝐾
i. Using the equation PV=nRT and STP (T=273 K, V=22.4L, n=1.0 moles) calculate the gas law
constant for the following units:
1. atm:
2.
Pa and kPa:
3.
mm Hg and torr:
4.
lb/in2 (psi):
g.
PV=nRT is used for single state situation. If you have a two state situation, you can solve both PV=nRT
equations for R (the constant) and set them equal.
h.
Practice Problems
i. What is the pressure (in atm.) of a gas if 2.00 moles occupy a volume of 10.0 L at 27.0ºC?
ii. A 2000.0 mL flask contains a gas under a pressure of 3800.0 mm Hg at 30.0ºC. How many
moles of gas are in the flask?
i.
Ideal gas versus non-ideal gas:
ideal:
non-ideal:
3
3.
Boyle’s Law
a. Boyle’s Law focuses on –
b.
Let’s start from the two state equation:
c.
If the temperature and number of moles remain constant, then _______________ and _______________.
d.
The two state equation now becomes:
e.
Boyle’s Law states –
i. 𝑉 𝛼 1⁄𝑃
f.
{
Hand pump:
i. A cylinder that when you pull the handle up the volume _______________ and the internal
pressure _______________ which draws the air into the pump.
ii. Then when you push the handle down the volume _______________ and the internal pressure
_______________ forcing the air out of the pump.
g.
Scuba Divers:
i. At sea level the pressure is 1 atm. For every 10 meters you descend, the pressure increases 1
atm. A diver diving to 20 meters will experience _____ atm.
ii. Pressure regulators deliver air that matches the external pressure. If this did not happen divers
______________________________________.
iii. If a person swam quickly to the surface with 3 atm. of pressure in their lungs, the lungs will
expand as the pressure drops because ______________________________________.
h.
Extra Long Snorkels:
i. If you were to use a long snorkel, about 10 meters, the pressure of your lungs 10 meters below
the surface is ____________.
ii. Since the pressure in your lungs at 10 meters is ____________ and the pressure where air is, is
____________, the air would flow __________________ their lungs making it
______________________ to breathe.
i.
Practice Problems:
i. Calculate the pressure of a gas that was initially at 765 mm Hg and 1.78 L and later compressed
to 1.25 L.
ii. A cylinder equipped with a moveable piston has an applied pressure of 4.0 atm. and a volume of
6.0 L. What is the volume of the cylinder if the applied pressure is decreased to 1.0 atm.?
4
4.
Charles’ Law
a. Charles’ Law focuses on –
b.
Let’s start from the two state equation:
c.
If the pressure and number of moles remain constant, then _______________ and _______________.
d.
The two state equation now becomes:
e.
Charles’ Law states –
i. 𝑉 𝛼 𝑇
f.
{
Hot air balloon:
i. Hot air _______________ because the volume of a gas at constant pressure _______________
with _______________ temperatures.
ii. Warming _______________ density because density equals mass divided by volume and the
volume is _______________.
iii. Lower density gas _______________ in higher density gas.
g.
Balloons:
i. If you blow up a balloon and put it in hot water it will _______________. ( _______________ )
ii. If you put the balloon in cool water it will _______________. ( _________________________ )
h.
Practice Problems:
i. If the temperature of 2.00 L of a gas at 50.0 ºC is increased to 100.0 ºC, calculate the new
volume.
ii. A sample of gas has a volume of 2.80 L at an unknown temperature. When the sample is put into
ice water at 0 ºC and its’ volume drops to 2.57 L, what was the initial temperature in K and ºC?
5.
Gay-Lussac’s
a. Gay-Lussac’s Law focuses on –
b.
Let’s start from the two state equation:
c.
If the volume and number of moles remain constant, then _______________ and _______________.
d.
The two state equation now becomes:
e.
Gay-Lussac’s Law states –
i. 𝑃 𝛼 𝑇
f.
{
Practice Problems:
i. If the temperature of 2.00 atm. of gas at 50.0 ºC increases to 100.0 ºC, calculate the new pressure.
ii. A sample of gas has a pressure of 883 mm Hg at an unknown temperature. When the sample is
put into ice water at 0 ºC, its’ pressure drops to 648 mm Hg, what was the initial temperature in K
and ºC?
5
6.
Combined Law
a. The combined gas law combines –
b.
Let’s start from the two state equation:
c.
If the number of moles remain constant, then _______________.
d.
The two state equation now becomes:
e.
Practice Problems:
i. A certain gas sample has a volume of 25.0 mL at 20.0 ºC and 735 mm Hg. What will the volume
of this gas be at 100.0 ºC and 800.0 mm Hg?
ii. A sample of gas has an initial volume of 158 mL at a pressure of 735 mm Hg and a temperature
of 34 ºC. If the gas is compressed to a volume of 108 mL and heated to a temperature of 85 ºC,
what is the final pressure in mm Hg?
7.
Avogadro’s Law
a. Avogadro’s Law focuses on –
b.
Let’s start from the two state equation:
c.
If the pressure and temperature remain constant, then _______________ and _______________.
d.
The two state equation now becomes:
e.
Avogadro’s Law states –
i. 𝑉 𝛼 𝑛
f.
{
Balloon:
i. When you blow up a balloon you _____________ the number of gas molecules in the balloon
and the volume _____________.
g.
Practice Problems:
i. A 4.8 L sample of helium gas contains 0.22 moles of helium. How many additional moles of
helium gas must be added to the sample to obtain a volume of 6.4 L?
ii. A chemical reaction occurring in a cylinder equipped with a moveable piston produces 0.58
moles of a gaseous product. If the cylinder contained 0.11 moles of gas before the reaction and
had an initial volume of 2.1 L, what was the volume after the reaction?
8.
Partial Pressures
a. Partial pressure is the pressure due to any individual component in a gas mixture.
b.
(Partial pressure of the component) = (fractional composition of the component) x (total pressure)
6
c.
Using the table, what is the partial pressure of nitrogen gas and oxygen gas if the total pressure is 1.0
atm.?
d.
Dalton’s Law of Partial Pressure – the sum of the partial pressures of each of the components in a gas
mixture must equal the total pressure.
e.
Deep Sea Diving:
i. If you are 30 meters below sea level (under water), the pressure is at _____________. If 1 atm is
equivalent to 0.21 atm. of oxygen, at 30 meters underwater, the partial pressure of oxygen is at
_____________.
ii. __________________________ – increased oxygen concentration leads to muscle twitching,
tunnel vision, and convulsions.
f.
Mountain Climbing:
i. On top of Mount Everest the pressure is 0.311 atm., what is the partial pressure of oxygen if at 1
atm. the partial pressure is 0.21 atm?
ii. __________________ – low oxygen levels lead to dizziness, headache, and shortness of breath.
g.
Practice Problems:
i. A mixture of helium, neon, and argon has a total pressure of 558 mm Hg. If the partial pressure
of helium is 341 mm Hg and neon is 112 mm Hg, what is the partial pressure of argon in the
mixture?
ii. If nitrogen gas makes up 78%, oxygen gas makes up 21%, and argon gas makes up 1% of air,
what are the partial pressures of each gas in air at 760 mm Hg?
9.
Gases in Chemical Reactions
a. Now we can combine the gas laws with stoichiometry and convert between grams and moles of one
substance, then to moles of another, and then use PV=nRT to solve for pressure, volume, or temperature.
b.
Practice Problems:
i. How many moles of NH3 are formed by the complete reaction of 2.5 L of hydrogen gas at 381 K
and 1.32 atm?
3H2 (g) + N2 (g) → 2NH3 (g)
ii. How many liters of oxygen gas form when 294 grams of KClO3 completely react in the following
reaction if the pressure is 755 mm Hg and the temperature is 305K?
2 KClO3 (s) → 2 KCl (s) + 3 O2 (g)
7
10. Graham’s Law
a. Graham’s Law states –
b.
Diffusion –
c.
Effusion –
d.
The single state equation is:
e.
The two state equation is:
f.
Practice Problems:
i. A compound effuses through a porous cylinder 3.20 times faster than carbon dioixde. What is
the molar mass of the compound?
ii. Gas A effuses at a rate of 0.68 times greater than gas B. If the mass of B is 17 grams, what is the
mass of gas A?
8








