Chapter 01
advertisement
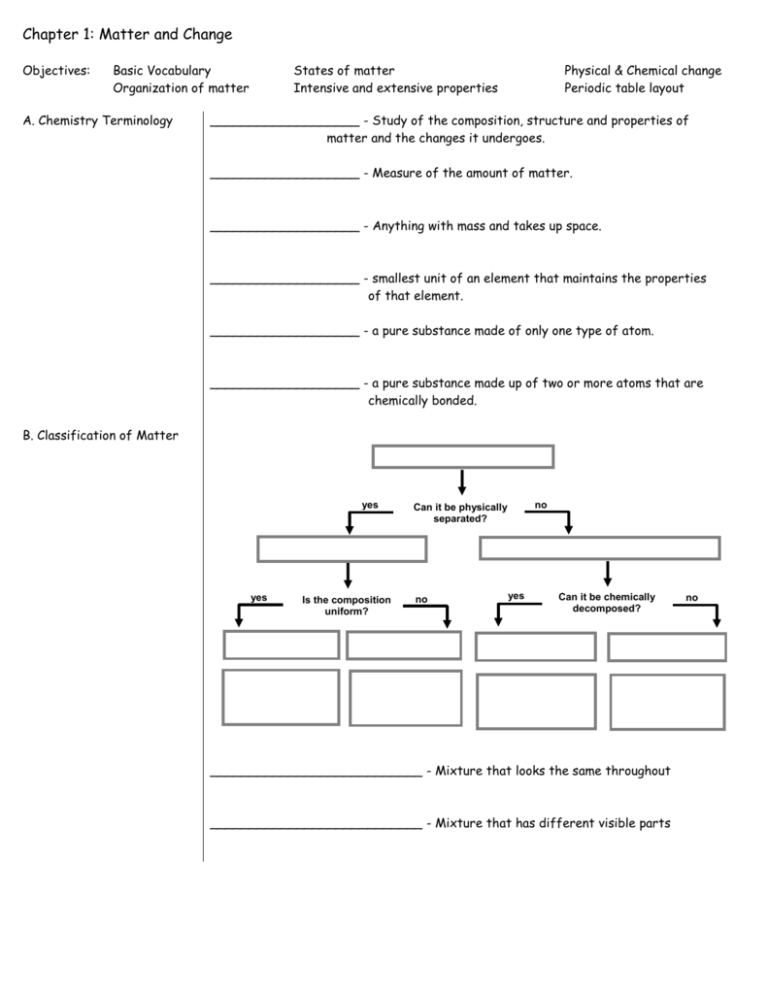
Chapter 1: Matter and Change Objectives: Basic Vocabulary Organization of matter A. Chemistry Terminology States of matter Intensive and extensive properties Physical & Chemical change Periodic table layout ___________________ - Study of the composition, structure and properties of matter and the changes it undergoes. ___________________ - Measure of the amount of matter. ___________________ - Anything with mass and takes up space. ___________________ - smallest unit of an element that maintains the properties of that element. ___________________ - a pure substance made of only one type of atom. ___________________ - a pure substance made up of two or more atoms that are chemically bonded. B. Classification of Matter yes yes Is the composition uniform? no Can it be physically separated? no yes Can it be chemically decomposed? ___________________________ - Mixture that looks the same throughout ___________________________ - Mixture that has different visible parts no C. Phases of Matter Bose-Einstein Condensate Solids Liquids Gases Plasma D. Extensive vs. Intensive _______________ - Depends on the amount of matter _______________ - Does not depend on the amount of matter Examples: Boiling Point Conductivity Mass Density Volume E. Chemical and Physical Properties __________________ - can be observed without changing the identity of the substance __________________ - describes the ability of a substance to undergo changes in identity Examples: Melting point Conductivity Flammability Tarnishes in air Magnetic E. Chemical and Physical Change _______________ * Changes the form of a substance without changing its identity * Properties remain the same _______________ * Changes the identity of a substance * Products have different properties Examples: rusting iron melting ice dissolving in water grinding spices burning a log F. Periodic Table A– B– C- C Carbon 6 12.011 1s2 2s2 2p2 The rows across are called _______________ The columns down are called ______________ There are several areas you should become familiar with: Family 1 is the _________________________ Area s is ________ Family 2 is the _________________________ Area p is ________ Area 3 is the ___________________________ Area d is ________ Family 4 is the _________________________ Area f is ________ Family 5 is the __________________________ Row 6 is the __________________________ Row 7 is the __________________________ Area 8 is the __________________________ From page 16: Know these 11 names for Elements Quiz Modern Name Antimony Copper Gold Iron Lead Mercury Potassium Silver Sodium Tin Tungsten Symbol Sb Cu Au Fe Pb Hg K Ag Na Sn W Latin Name Stibium Cupprum Aurrum Ferrum Plumbum Hydrargyrum Kalium Argentum Natrium Stannum Wolfram (German)