Oral Presentation 5
advertisement

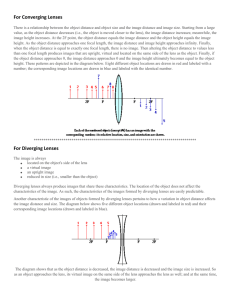
Transition Contacts in Action Paavan Kotini, Mike Matthews, Raj Ganguly, Sam Leu, Jung Lee The need-Coming Outside The need- Military The need- Athletes • At time uv light can impair actions. The goal of this project is to use previous incumbent technology of transition glasses and apply it to contacts. General Mechanism of Photochromism • • • • mechanism over the course of the colour change, the photochromic molecule represented simplistically as two rings. Upon the action of UV light or direct sunlight, the structure twists from a perpendicular (closed) form to a flat, planar (opened) structure. This allows the two halves to interact, resulting in the absorption of visible light. There are effectively two changes occurring simultaneously; a chemical change arises when the molecule is exposed to UV light, that enables conjugation to take place throughout the molecule; a structural change also occurs to enable the overlap of ∏-orbitals. Therefore, spatially, the molecules must be able to flatten out to allow this conjugation to take place. It is a fully reversible reaction so that when the light source is removed, the molecule returns to its uncoloured state. Heat can also help drive the reaction back to the uncoloured form, so in very hot conditions, there is always competition between light and heat to determine the given colour observed. In general, a colour change is still observed, albeit weaker than at room temperature. Similarly, in cold conditions in the presence of sunlight, an intense colour is observed as there is little or no competition from the back reaction. • • Kinetics This is a cyclic reaction and the number of cycles (or the activation and fade rates) varies greatly by product. The activation times are generally much shorter than fade times. On average, fade times are two or three times longer than activation times. The Reversacol product range offers a very large variation in kinetics characteristics. Some fade in several seconds, whilst others can take several minutes. • • Systems As well as the nature of the product influencing the colour and kinetics result, the system or matrix used with the dye has a strong influence on such properties. For example, in some systems, a colour shift of up to 20nm has been observed. http://www.photochromics.co.uk James Robinson Reversacol Photochromic Dyes Market Research • Current solutions include: sunglasses, contact lenses with UV protection, and transitioning eyeglasses. • Each confers benefits to the user, but fails to bridge the gap in technology for low and high intensity situations; or provide an optimum level of convenience. Market Research Vision-Corrected Market by Year vs. U.S. Population (Millions) Source: Health Products Research, Inc • Since 2002, market reports (e.g Optistock.com) show that the demand for contact lenses has been growing; especially specialty contacts, which currently account for one-third of the world demand for contact lenses. • Unfortunately these specialty contacts fail to reduce glare in the user’s eyes, making it necessary to use other sun light intensity reducing technologies (e.g sunglasses). Market Research Nike MAXSIGHT™ lenses by Bausch & Lomb Source: http://www.nike.com/nikebiz/nikebiz.jhtml?page=2&item=maxsight http://www.bausch.com/us/vision/products/softcontacts/nikemaxsight_faq.jsp http://www.flinthillseyecare.com/pdf/Maxsight_Nike_CL.pdf#search='nike%20maxsight' Market Research • Possible Cost for production of transition contact lens • Selling price of product would be between $40 and $60 dollars for 2 boxes of Contact Lens PMMA Polymethacylate • • • PMMA or polymethylmethacrylate is a clear plastic vinyl polymer that is more transparent than glass. PMMA has been used safely for almost 50 years for contact lenses. Advantages – – – • Studies with spiropyrans and spiro-oxazines show that it does not slow down UV response times of Photochromic pigments. It is also water soluble It is able to readily bind with the pigment Disadvantages – – not very gas permeable( very low oxygen penetration) low elasticity Silicon Hydro Gel • An inorganic matrix of silicon dioxide. • Used in creating current soft contact lenses • Advantages – It allows the contact lens to have greater flexibility – Allows for greater oxygen penetration • Disadvantages – Is not very compatible with the photochromic pigment • Slows down transition time • The binding process is complicated Poly-HEMA • Used as backbone for many hydrophilic contact lenses • 1st to be used in hydrophilic contact lenses • Very stable and safe material once polymerised Polydimethylsiloxane (PDMS) Elastomer • [SiO(CH3)2] • Widely used siliconbased organic polymer • Optically clear, considered to be inert, non-toxic and non-flammable 1′,3′-Dihydro-8-methoxy-1′,3′,3′-trimethyl-6nitrospiro[2H-1-benzopyran-2,2′-(2H)indole] •Type: Spiro-pyran •Molecular Weight: 352.4 g/mol •Melting Point: 159 – 162 °C •Price: $40.50 per 1g (Sigma Aldrich) 1′,3′-Dihydro-1′,3′,3′-trimethyl-6-nitrospiro[2H-1benzopyran-2,2′-(2H)-indole] • • • • Type: Spiro-pyran Molecular Weight: 322.36 g/mol Melting Point: 179-180 °C Price: $68.40 per 1g (Sigma Aldrich) Toluene • Appearance: Colourless liquid • Melting point: -93 C • Toxicology– Toxic by inhalation, ingestion or absorption through skin. Serious irritant • Widely use as a solvent • Evaporates during procces of making contact Process of Making Contact Lens • PDMS monomer to Curing Agent • 1 Monomer : 5 Curing Agent • Mix Monomers with Curing Agent Chemical in PDMS Soluble Test • Dissolve chemical with toluene • • • • 0.5 mg of Chemical 400 µl of toluene ≈0.3 ml of PDMS ≈0.066 ml of Curing Agent Surface Coating • Make PDMS • ≈0.5 mg of Chemical • 100 µl of toluene Chemical Vapor Deposition Concentration Experiment • Pigment 1 • 0.001, Mold Making • Contact Vanderbilt Machine Shop • VIIBRE – Use Epoxy – Use Crystal Clear • Design Usage – mass production of contact lens Mixing of two Chemicals Future Direction 1. Radial limiting of pigment deposition 2. Safety, will be testing leak rate in saline solution. 3. Safety in terms of lesions, eye irritation, and chemical reactions in live animal eyes 4. Human testing via FDA approval 5. Manufacture Demonstration Contact Lens d= 30mm (Area ~ 9 X of normal contact lens) References • http://www.contamac.com/db/display/glossary • http://www.bimax.com/hema.htm • http://www.polymer.org.au/uqchem/hydrogels.html • http://chemweb.calpoly.edu/chem/gragson/Teaching/chem354/Lab_instructions/Photo chromism.pdf#search='Spiropyrans' • http://www.thieme-connect.com/ejournals/pdf/synfacts/doi/10.1055/s-2005869881.pdf#search='Spiropyrans' • http://www.freepatentsonline.com/4826977.html (Photochromic spiropyran compounds , United States Patent 4826977) • http://pubs.acs.org/cgi-bin/abstract.cgi/jpchax/1972/76/i24/fpdf/f_/j100668a007.pdf?sessid=6006l3 • http://www.photochromics.co.uk/index.htm References • Lay Gaik Teoha, Jiann Shiehb, Wei Hao Laia, I Ming Hunga, Min Hsiung Hon. “Structure and optical properties of mesoporous tungsten oxide.” Journal of Alloys and Compounds. (2005) 251–254 • Robert G. Palgrave and Ivan P. Parkin. “Aerosol assisted chemical vapour deposition of photochromic tungsten oxide and doped tungsten oxide thin films.” Journal of Materials Chemistry. 2004, 14, 2864 – 2867 • http://www.freepatentsonline.com/5458815.pdf • S. Delbaere,a, J.-C. Micheau,b Y. Teral,c C. Bochu,a M. Campredon, c and G. Vermeerscha. “NMR Structural and Kinetic Assignment of Fluoro-3H-naphthopyran Photomerocyanines.” • • www. Alcok.com/intac.html Andersson, Nina, Alberius, Peter, Ortegren, Jonas, Lindgren, Mikael, and Bergstrom, Lennart. “Photochromic Mesostructured Silica Pigments Dispersed in Latex Films”