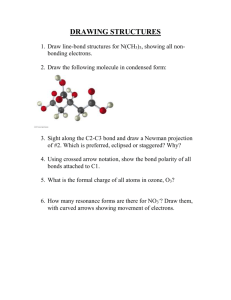
Above is a caffeine molecule. Label all of the atoms as sp, sp2, or
advertisement
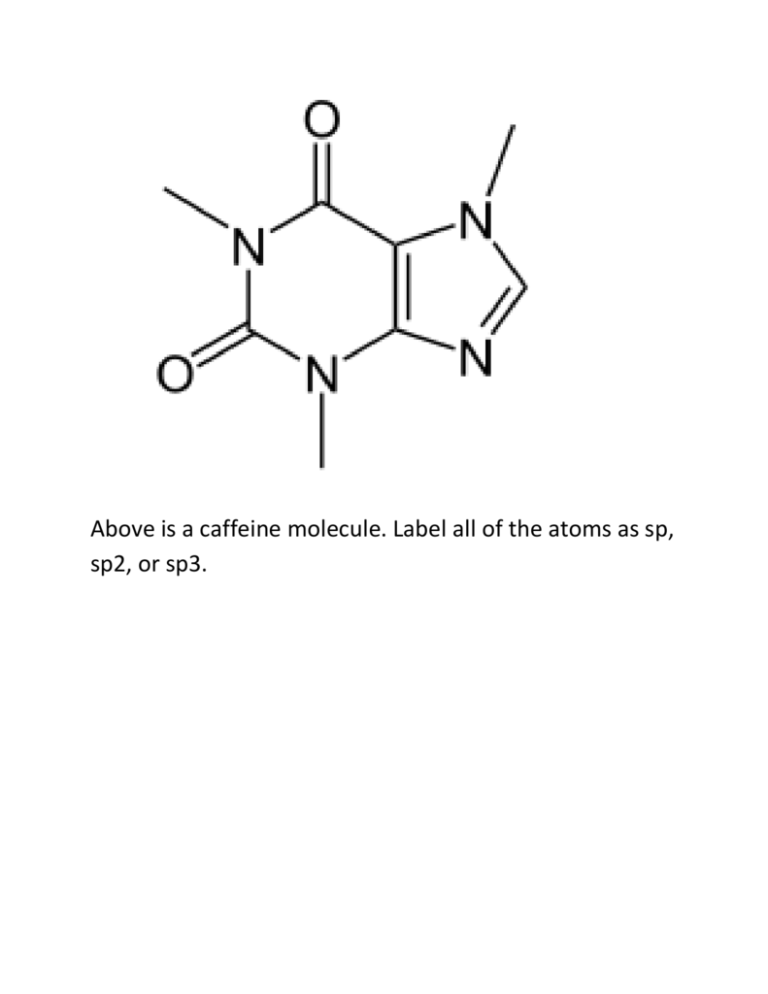
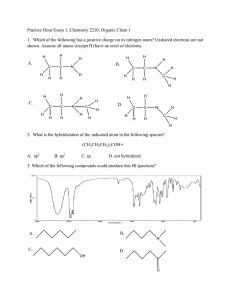
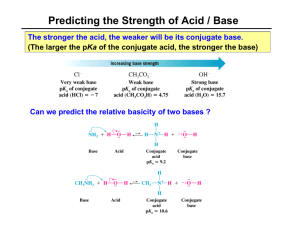
Above is a caffeine molecule. Label all of the atoms as sp, sp2, or sp3. What is the electron configuration for Bromine? Draw the two resonance structures of ozone, O3. Write what type of hybridization each carbon has. Draw the electron cloud for each bond of this molecule. Draw a corrected version of this molecule, including formal charges. Vinegar and sodium hydroxide react. Show the reaction, the transfer of electrons using arrows, label the conjugate acid and conjugate bases, and predict the direction of the reaction using the pKa’s. The pKa of acetic acid is 4.75 and the pKa of water is 15.7. Identify the following as Lewis acids/bases and Bronsted Lowry acids/bases: BF3 NaOH Methoxide HCl Acetic acid Draw all of the resonance structures for these three molecules. Draw 1-chloropropane as a sawhorse and all the newman projections. Draw gauche, the other staggered conformations, and the eclipsed conformation. There should be a total of four at different energy levels. Which are the highest and lowest in energy? Draw the following alkyl groups: Methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl Draw examples of London dispersion forces, dipoledipole forces, and hydrogen bonds with ammonia. What are the requirements for hydrogen bonding? Draw an example of the following functional groups: Arene, Alkane, alkene, alkyne, alcohol, ether, ester, carboxylic acid, amide, ketone, aldehyde Name this molecule: Draw 1,1-dibromo-3,5-dichloro-4-isopropylpentane. Then write its correct name and why the original name is wrong. Draw C4H8O, with an alcohol group Draw C5H6BrClO, with a ring Draw 2,2-dimethylpentane with stereochemistry (dashes and wedges) Find the relations between these compounds (constitutional isomers, identical molecules, and not isomers): Give one possible structure for the following formula, using the IR spectra to determine the functional group. C4H10O