4_uses_of_ideal_gas_..
advertisement
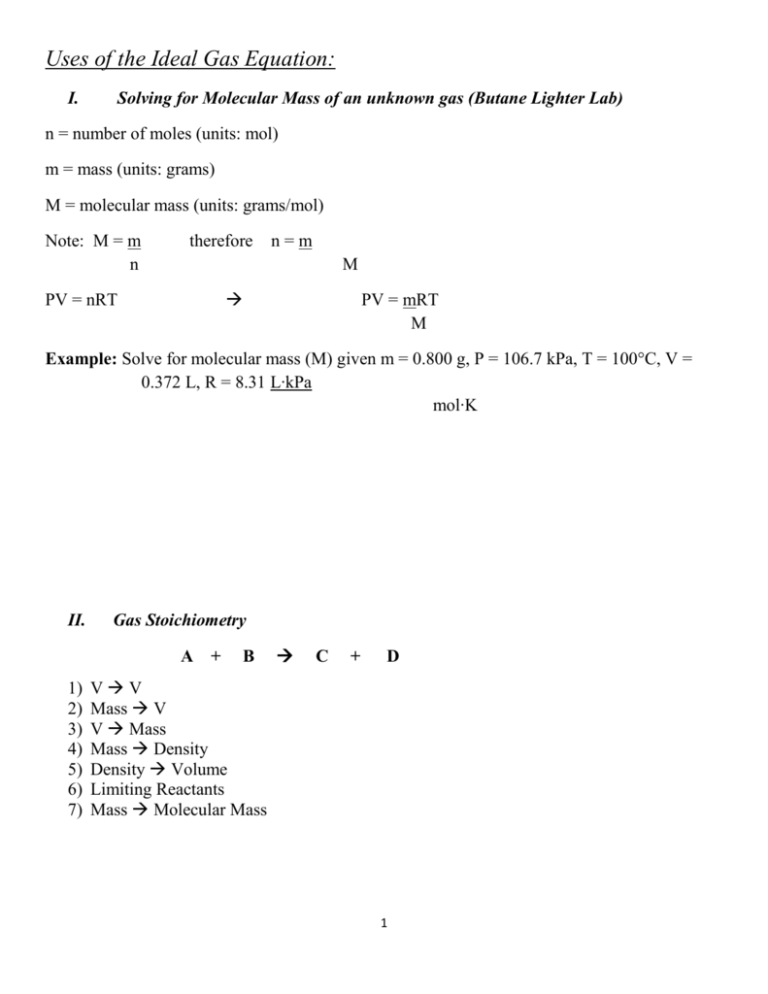
Uses of the Ideal Gas Equation: I. Solving for Molecular Mass of an unknown gas (Butane Lighter Lab) n = number of moles (units: mol) m = mass (units: grams) M = molecular mass (units: grams/mol) Note: M = m n therefore n=m M PV = nRT PV = mRT M Example: Solve for molecular mass (M) given m = 0.800 g, P = 106.7 kPa, T = 100°C, V = 0.372 L, R = 8.31 L∙kPa mol∙K II. Gas Stoichiometry A + 1) 2) 3) 4) 5) 6) 7) B C + D VV Mass V V Mass Mass Density Density Volume Limiting Reactants Mass Molecular Mass 1 Example 1: Volume to Volume Stoichiometry Gas Law Problem What volume of gas is produced from x liters substance? How many liters of hydrogen chloride gas will be produced from 2.00 L hydrogen gas? H2 (g) + Cl2(g) 2 HCl (g) 2.00 L ?? L Example 2: Mass to Volume Stoichiometry Gas Law Problem What volume of gas is produced from x grams substance? Steps: 1. Balance equation and compound formulas 2. Find moles 3. Find mole ratio 4. Convert moles gas volume of gas (1 mol = 22.4 L) How many liters of hydrogen gas will be produced from 6.54 g of zinc? 2 HCl (aq) + Zn (s) H2 (g) + ZnCl2 (aq) 6.54 g ?? L Example 3: Volume to Mass Stoichiometry Gas Law Problem What mass of gas is produced from x liters substance? What mass of magnesium will react with excess sulfuric acid to produce 500 mL of hydrogen? Mg ?? g + H2SO4 H2 + MgSO4 500 mL 2 III. Determining Gas Density Step 1: Start with PV = mRT and solve for the molecular mass (M). M Step 2: Density (D) is mass (m) per unit volume (V). Write in equation form: ________________ Step 3: You can introduce the density equation into the equation from step 1. Find m in M = mRT equation from step 1. Replace m with D. PV V Step 4: Solve for density (D). Example 1: Calculate the density of sulfur dioxide at STP to 3 significant figures. (hint: from balloon worksheet) (Ans: 2.87 g/L) Example 2: What is the density of a sample of ammonia gas, NH3, if the pressure is 0.928 atm and the temperature is 63.0°C? (Ans: 0.527 g/L) 3 Molar Volume Problem Example 11-2 A reaction yields 98.0 ml of SO2 gas at STP. What is the mass (g) of the gas produced? Answer: 0.280 g SO2 0.098 L 1.0 mol SO2 22.4 L 64.1 g SO2 = 0.280 g SO2 1 mol SO2 (Ex. 2) 4.37 g of a diatomic gas occupies a volume of 3.00 L at 1.00 atm and T=45oC. What is the gas? F2 4