File
advertisement

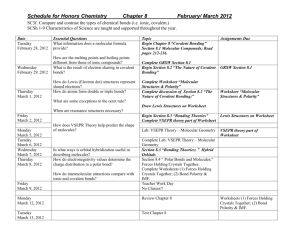
Honors Chemistry Unit 3: Chemical Bonding and Nomenclature Introduction: The elements we have been studying are often called “the building blocks of the Universe”. Elements in their pure form, however, have a somewhat limited usefulness. It is only when you start putting the building blocks together in varying patterns that chemistry becomes really interesting. As is the case with all of nature, the putting together of these blocks is governed by certain rules which must be understood before chemistry, as a whole, can be understood. It has taken almost two centuries of diligent work to uncover what we not know about the rules of chemical bonding. The approximately two million compounds that we have been able to synthesize so far, however, demonstrates a fair grasp of these rules, which we will discuss. This unit concerns itself mainly with the rules governing the joining of two or more atoms to form a chemical bond. Since chemical bonding lies at the very heart of the study of chemistry it is a very important unit. Learn it well! We will cover two chapters in this unit: chapters 5 and 10. There is a lot of information so you must work in and outside of class to stay on top of the information. Major Objectives: When you have completed this unit you will be able to: 1. 2. 3. 4. 5. 6. 7. 8. 9. Define ionic, covalent, metallic, and coordinate covalent bonding and explain the differences between them Write the correct formula for a compound, given the scientific name and a table of names, formulas, and charges of most common ions. Write the correct name for a compound given its formula and a table of names, formulas, and charges of most common ions. Construct a Lewis (electron-dot) structure of any simple compound given its formula. Predict the most probable type of bonding in a compound, given its formula and a table of electronegativities. Calculate the molecular mass of a compound, given its formula. Use the VSEPR theory to predict the shapes and geometry of compounds Name and write formulas of acids Use the geometry of a molecule to predict its polarity Required work for Unit 5 Vocabulary: When you have finished this unit, you will be able to define and to use the following words and phrases as part of your working chemistry vocabulary: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. Ionic compound Ionic bond Chemical formula Formula unit Valence electron Lewis dot structures Monatomic ions Polyatomic ions Molecular compound Covalent bond Molecule Diatomic molecule Molecular formula Single covalent bond Structural formula Unshared pair Double covalent bond Triple covalent bond Bond dissociation energy 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. Resonance structure Binary compound Coordinate covalent bond Molecular/ molar mass Law of definite proportions Law of multiple proportions Hydrates Acid Bent Bonding theory Bonding pair Linear Molecular geometry Tetrahedral Trigonal planar Trigonal pyramidal VSEPR theory Assigned reading: Be sure to read the required reading in your textbook: Pearson Chemistry, Indiana ed. 1. Chapter 5: P 127 – 152 2. Chapter 10: P325 – 348 Assignments: 1. 2. 3. 4. 5. 6. 7. 8. Introduction to Ionic Bonding worksheet Lets practice: Binary Ionic compounds worksheet Make ‘em Happy worksheet Hodgepodge Homework sheet Lewis Structure worksheet Naming Covalent Compounds worksheet VSEPR theory worksheet Naming acids Labs: 1. Covalent Bonding in Molecular Compounds/VSEPR theory