File
advertisement

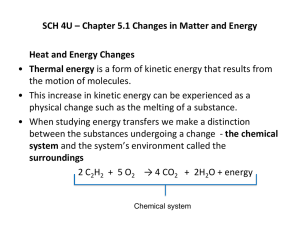
Energy balance in steady State System All energy-balance calculations are based on the law of conservation of energy, which states that energy can be neither created nor destroyed. Although this law does not apply to nuclear reactions, conservation of energy remains a valid principle for bioprocesses because nuclear rearrangements are not involved. The law of conservation of energy can be written as: ……………..1 Fig: Flow system for energy-balance calculation In the above figure: Mi is the mass enters into the system while mass Mo leaves. Both these masses have energy associated with them in the form of internal, kinetic and potential energy; flow work is also being done. Q is the energy leaves the system as heat Ws is shaft work is done on the system by the surroundings. We will assume that the system is homogeneous without charge or surface-energy effects. To apply Eq. (1), we must identify which forms of energy are involved in each term of the expression. If we group together the extensive properties and express them as specific variables multiplied by mass, Eq. (1) can be written: …………………2 where subscripts i and o refer to inlet and outlet conditions, respectively, and AE represents the total change or accumulation of energy in the system, u is specific internal energy, e k is specific kinetic energy, e is specific potential enerev, p is p i 1 c:gJ pressure, and v is specific volume. All energies associated with masses crossing the system boundary are added together; the energy-transfer terms Q and W are considered separately. Shaft work appears explicitly in Eq. (5.5) as Wss; flow work done the by inlet and outlet streams is represented as pv multiplied by mass. Energy flows represented by Q and W can be directed either into or out of the system; appropriate signs must be used to indicate the direction of flow. Because it is usual in bioprocesses that shaft work be done on the system by external sources, in this text we will adopt the convention that work is positive when energy flows from the surroundings to the system as shown in Figure 5.1. Conversely, work will be considered negative when the system supplies work energy to the surroundings. On the other hand, we will regard heat as positive when the surroundings receives energy from the system, i.e. when the temperature of the system is higher than the surroundings. Therefore, when W s and Q are positive quantities, W s makes a positive contribution to the energy content of the system while Q causes a reduction. These effects are accounted for in Eq. (5.5) by the signs preceding Qand W s. The opposite sign convention is sometimes used in thermodynamics texts. Choice of sign convention is arbitrary if used consistently. Eq. (5.5) refers to a process with only one input and one output stream. A more general equation is Eq. (5.6), which can be used for any number of separate material flows: The symbol E means summation; the internal, kinetic, potential and flow work energies associated with all output streams are added together and subtracted from the sum for all input streams. Eq. (5.6) is a basic form of the first law of thermodynamics, a simple mathematical expression of the law of conservation ofenergy. The equation can be shortened by substituting enthalpy h for u + pv as defined by Eq. (5.3): Special Cases Eq. (5.7) can be simplified considerably if the following assumptions are made: (i) kinetic energy is negligible; and (ii) potential energy is negligible. These assumptions are acceptable for bioprocesses, in which high-velocity motion and large changes in height or electromagnetic field do not generally occur. Thus, the energy-balance equation becomes: Eq. (5.8) can be simplified further in the following special cases: (i) Steady-stateflow process. At steady state, all properties of the system are invariant. Therefore, there can be no accumulation or change in the energy of the system: AE = 0. The steady-state energy-balance equation is: Eq. (5.9) can also be applied over the entire duration of batch and fed-batch processes if there is no energy accumulation; 'output streams' in this case refers to the harvesting of all mass in the system at the end of the process. Eq. (5.9) is used frequently in bioprocess energy balances. (ii) Adiabaticprocess. A process in which no heat is transferred to or from the system is termed adiabatic; if the system has an adiabatic wall it cannot receive or release heat to the surroundings. Under these conditions Q- 0 and Eq. (5.8) becomes: Eqs (5.8)-(5.10) are energy-balance equations which allow us to predict, for example, how much heat must be removed from a fermenter to maintain optimum conditions, or the effect of evaporation on cooling requirements. To apply the equations we must know the specific enthalpy h of flow streams entering or leaving the system. Methods for calculating enthalpy are outlined in the following sections.