saponification for class
advertisement
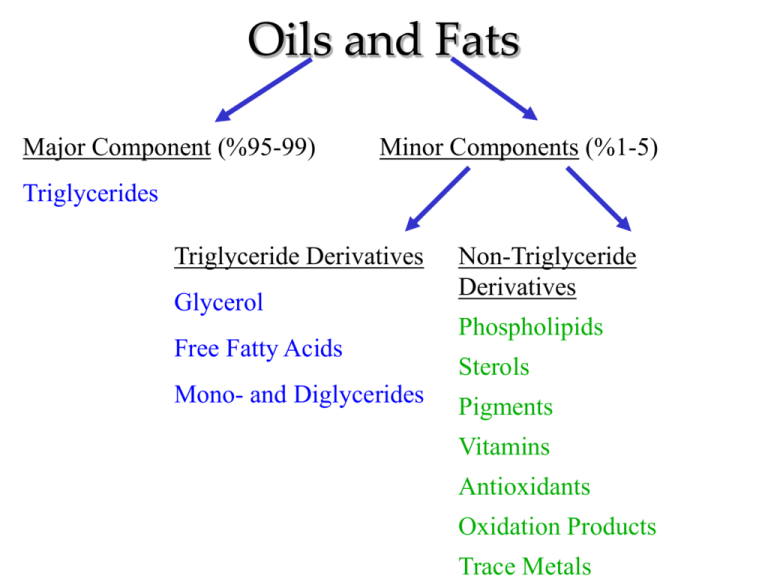
Oils and Fats Major Component (%95-99) Minor Components (%1-5) Triglycerides Triglyceride Derivatives Glycerol Free Fatty Acids Mono- and Diglycerides Non-Triglyceride Derivatives Phospholipids Sterols Pigments Vitamins Antioxidants Oxidation Products Trace Metals Triglyceride Structure H2C OH 3 fatty acids O O O C - R1 O O HO - C - R1 HC OH + = HC O C- R2 +3H20 HO - C - R2 O H2C OH O glycerol H2C O C - R3 HO - C - R3 triacylglycerol H2C –One chiral carbon with 1-3 acyl groups –simpler stereochemistry than sugars –more possible substituents O -C R acyl Saponification General reaction Fat + Base Soap + glycerine Base = chemical that contains OH at the end Creating soap from fats or oils. Soaps are usually made from vegetable fats and oils. These consist of 3 fatty acid chains, held together by a glycerol molecule. Fatty acid glycerine Triglycerides differ from each other in regard to • Number of Carbon Atoms in fatty acid chains • Number of double bonds • Isomerization • Distribution of FA on glycerol backbone How are soaps made? This reaction is basically the opposite of an esterification Esterification – remove HOH to connect molecules Saponification – Add NaOH to break molecules Esterification H HO Saponification Na HO What is a soap? Because a soap molecule has a long carbon chain, it is partially nonpolar. Nonpolar - + Polar Na Attracts oil Attracts water The sodium part has a + and – charge, so it’s polar at this end. On end can attract polar molecules like water (hydrophilic), the other end can attract nonpolar molecules like oil (hydrophobic) Saponification Reaction: Fat + Lye Soap + Glycerol Lye - Caustic solution made from ashes. Soda Lye = NaOH Potash Lye = KOH Combining potash K2CO3 with slaked lime Ca(OH)2 yields KOH and CaCO3 NaOH obtained from reacting sodium compounds. O H C17H35-C-O-C-H O C17H35-C-O-C-H O C17H35-C-O-C-H H Fat (Triglyceride) + NaOH + NaOH + NaOH Lye O C17H35-C-ONa O C17H35-C-ONa O C17H35-C-ONa Soap! H H-C-OH + H-C-OH H-C-OH H Glycerol How does it happen?? O • Break the ester linkages C – O- by hydrolyzing the bonds between the carbon backbone and the fatty acid chains. • (Reversal of esterification reaction) • Form the sodium salt of a fatty acid (soap) and a trihydroxy alcohol (glycerol). The Chemistry http://en.wikipedia.org/wiki/Saponification If oil is added to water, the two liquids do not mix. Because of this, grease stains can be difficult to remove during washing. Soaps are compounds which act as emulsifiers. soap molecule water This means that they help the oil to mix with the water. oil How do soaps work? • Organic Reaction: Slow! • Must break covalent bonds. • Refluxing: Technique which allows a volatile solvent to boil, condense and return to the reaction vessel. It helps to maintain a fairly constant temperature throughout the reaction period. Strange but true… Fat in cadavers: saponifies if conditions are right. Alkalai environment, moist, minimal oxygen. Adipocere or grave wax is formed. Saponification Value Saponification - hydrolysis of ester under alkaline condition. The saponification value of an oil or fat is defined as the number of mg of potassium hydroxide (KOH) required to neutralize the fatty acids resulting from the complete hydrolysis of 1 g of the sample. Saponification Value Determination Saponification # --mgs of KOH required to saponify 1 g of fat. 1. 5 g in 250 ml Erlenmeyer. 2. 50 ml KOH (0.5 N) in Erlenmeyer. 3. Boil for saponification. 4. Titrate with HCl (0.5 N) using phenolphthalein. 5. Conduct blank determination. SP# = 56.1(B - S) x N of HCl Gram of Sample B - ml of HCl required by Blank. S - ml of HCl required by Sample. N- Factor of 0.5 N HCL.