Surface Physics Gao Xingyu Office: S13 M01
advertisement

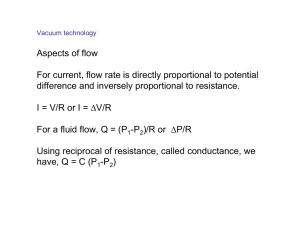
Topics in Surface Science Gao Xingyu Office: S13 M01-12 Tel: 6516 2970 (office) 6516 1670 (SSLS) http://www.physics.nus.edu.sg/~phygaoxy/ What is Surface? A special interface between solid/liquid and vacuum Interface: a small number of atomic layers that separate two solids in intimate contact with one another Why Surface? (do you know something interesting for surface?) •Many properties determined by the surface/interface •Many processes happens mainly at surface (chemical reaction including catalysis, crystal growth, thermionic emission) •Surface/interface may differ significantly from the bulk (Phase Density, Composition, Mechanical, Electronic, Magnetic properties…) •Strong interrelation of surface /interface with many other research fields •Surface as ultra-thin films presents 2-D model for theory (even 1D eg. Nanostructures - 3D thick film) •Many technical applications (semiconductor device) Surface can be unique !!! What determines the properties Atoms of which element? What structure? First principles calculation will deliver most important properties. Topics in Surface Science •Introduction vacuum technique, preparation of clean surface •Surface Chemical Composition AES, XPS, SIMS, XAS •Surface Morphology and Physical Structure surface tension, relaxation, reconstruction, and defects surface lattice Experimental Techniques: LEED, LEEM, RHEED, PEEM, XD, FIM, RBS/IS, SPM (STM, AFM, MFM), SEM, TEM, SEXAFS, PhD •Surface Electronic Structure Surface Potential and Work Function, surface states (intrinsic and extrinsic), band bending surface plasmons Experimental Techniques: PES (XPS, UPS), Inverse Photoemission, EELS, Kelvin Probe •Surface Magnetic Properties: some theoretical consideration some magnetic phenomena Experimental Techniques: VSM, MOKE, EMPA, SQUID, Ac susceptibility, XMCD, XMLD, spin-resolved photoemission, MDAD •Surface Vibrations Surface lattice dynamics Surface diffusion and surface melting Experimental Techniques: HREELS, Atom and Molecular Beam scattering, •Adsorption of atoms and molecules physisorption and chemisorption, Desorption techniques: Kelvin Probe, PES (XPS,UPS), surface segregation and epitaxial processes film growth techniques: MBE, MS, PLD Reference 1. 2. 3. 4. 5. 6. 7. 8. Surface and Interfaces of Solids by Hans, Luth Springer-Verlag 2001 (4th. Edition) Very good Concepts in Surface Physics by M.-C. Desjonqueres, D. Spanjaard, Springer , 1996 Introduction to Surface Physics (2nd Ed) by M. Prutton, Oxford Science Publications 1994 Good for newcomer Physics at Surfaces by A. Zangwill, Cambridge University Press 1988 Handbook of Surface Science by N. V. Richardson & S. Holloway North-Holland 1996 Vol. 1. Physical Structure Vol. 2. Electronic Structure Vol. 3. Dynamics Principles of Adsorption and Reaction on Solid Surfaces by Richard I. Masel Hohn, Wiley & Sons, Inc. 1996 Surface Science An Introduction by J. B. Hudson, John Wiley & Sons, Inc. 1998 Online source: http://www.uksaf.org/tutorials.html The way we mark your performance • 50% on final test • 35% on group work and workshop presentations about certain topics • 15% on tests in class Vacuum technology Rough (low) vacuum : 1 - 10-3 Torr Medium vacuum : 10-3 - 10-5 Torr High vacuum (HV) : 10-6 - 10-8 Torr Ultrahigh vacuum (UHV) : < 10-9 Torr (Pressure unit conversion) Why UHV is necessary? The incident flux is given by (atoms/m2s) m - molecular mass [ kg ], k - Boltzmann's constant ( = 1.38 x10-23 J K-1 ), T temperature [ K ], p = 3.1416. When it assumes unity "sticking coefficient“: Clean surface require UHV. Lots of techniques require UHV, electrons, soft x-ray, etc. UHV ensures that significant contamination by background gases does not occur during an experiment !!! Pressure (Torr) Gas Density (molecules m-3 ) Time / ML (s) 2 x 1025 10-9 10-3 3 x 1019 10-3 10-6 3 x 1016 1 10-10 3 x 1012 104 760 (ATM) UHV system chamber Connection pumps Stainless Flanges apparatuses vessel & tubes & valves manipulator Rough High vacuum UHV UHV system XPS chamber at SSLS Bellows Ion pump From back Rough pump Turbo pump What should do to get UHV? 1. 2. 3. 4. 5. 6. Right configuration Close all the flanges Make sure all the material inside clean and without incompatible materials Start pumping in the right sequence Proper Baking Degass all the filaments Heater and fan XPS chamber during During baking Construction Materials which are compatible with UHV •OFHC copper, also pure copper •Be-Cu alloy, phosphor bronze •304 SS (non-magnetic), 310 series SS, 340 SS (magnetic) •Teflon (gassy, compressible) •MACOR (machinable glass composite) •6061 Al (essentially pure aluminum), 2024 Al (harder alloy) •quartz, pyrex (gassy) •alumina (careful with glazed ceramics) •molybdenum, tungsten •"mu-metal" magnetic shielding (Co, Ni, Fe) •polyimide (Vespel) •Sn-Ag solder Materials which should be avoided •Zn, Cd: especially be careful of fasteners, bolts •Brass •Certain solders (eutectics have high vapor pressures Common vacuum problems 1. 2. 3. 4. 5. Improper cleaning techniques Incompatible materials Leaks Virtual leaks Not proper baking O-ring seals For low vacuum Metal knife-edge seals For UHV Pumps Rotary pump 2-stage one Basic one How it works Turbo pump Sorption pump Sublimation pump Ion getter pump Anode cathode cathode Cryopump High pumping speed Most gases except He and H2 Cannot be used above 10 torr Consume coolant as liquid N2 or He Normally used together with other pump Diffusion pump Vapor pressure determines the final pressure (depends on fluid) Require cooling water Robust, high speed, inexpensive and reliable Oil decomposition, dirty Gauges Thermocouple gauge Constant current heats filament Thermocouple measure T of filament Gas sensitive 10-10-4 torr Pirani gauge: The temperature change measured as resistance Gas sensitive 102-10-4 torr Ion gauge Ionization rate of the residual gas 10-4-10-11 torr How to find a leak? Simple ways for low vacuum leak 10-6-7 one can try acetone spray to check the variation of ion gauge pressure Most efficient: RGA (residual Gas analyzer) 1. filament and anode 2. Quadrupole 3. ion detector. Quadrupole is the essential part of RGA -(U+Vcos(ωt)) (U+Vcos(ωt)) How it works? Details here! There are two methods: varying ω and holding U and V constant, or varying U and V (U/V) fixed for a constant ω. Typical mass spectrum The mass spectra of the residual gas in the chamber tells essential information about the vacuum system (with water,…?) Helium gas was widely used to help RGA locate precisely the leak point Basic theory of Vacuum The final pressure of the chamber is determined by the outgassing rate of the chamber and the pumping speed. The pumping equation is vAv = Vv/kT(dp/dt + Sp/Vv) where Av and Vv is the inner surface area and volume of the chamber, respectively, Sp is the pumping speed and v is the outgassing rate. In the real cases, one need also consider C the finite conductance of the tubes through which the gas is pumped. C=I*RT/Dp I is the gas current, R is a constant and Dp is the pressure difference through the tube. For a single tube, C is only determined by mean free path length of the gas and the dimension of the tube. The tubes have a sum rules for conductance like the electric capacitors. The efficient pumping speed 1/Seff=1/S + 1/Cs How to prepare clean surface Cleavage in UHV: Many materials like some alkali halides (NaCl etc.), oxides (ZnO etc.),and some semiconductor (Ge, Si etc.) It happens along certain low index axis. Ion bombardment and Annealing: Surface bombarded by noble gas ion and then annealing with many cycles. One kind of sputter gun Heating: Temperature near melting point, for example Si(111) at 1370 K remove C and O Chemical processes: with acids such as HF and other chemical solution to make fresh surface then move into UHV Film growth: Grow new film with high purity in UHV condition using MBE, etc. How to check cleanness? Or how do you know the surface is clean (without contamination)? Chemical component analysis (with surface sensitivity)