Protein Structure/Function
advertisement

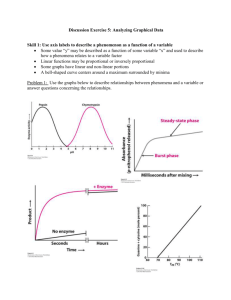
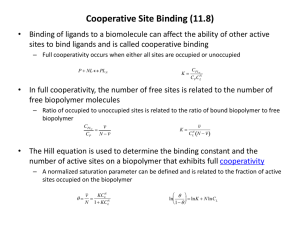
Protein Structure/Function C483 Spring 2013 1. Proteins segments which fold first can promote the folding of other sections of the protein into the native conformation by a process known as A) renaturation. B) stabilization. C) hydrophobic interaction. D) disulfide bridge formation. E) cooperativity. 2. Hydrophobic amino acid sequences in myoglobin are responsible for A) covalent bonding to the heme prosthetic group. B) the folding of the polypeptide chain. C) the irreversible binding of oxygen. D) A and B above. 3. A hyperbolic binding curve differs from a sigmoidal binding curve in that the hyperbolic curve A) has a single equilibrium constant for binding. B) always has a higher dissociate constant than a sigmoidal curve. C) shows cooperativity. D) suggests multiple ligand binding. E) All of the above. 4. Conditions in the tissues which enhance the delivery of oxygen by hemoglobin are the presence of A) more carbon dioxide present. B) 2,3 BPG present. C) lower pH. D) All of the above. E) A and B above. 5. True/False The hydrophobic crevice of globin prevents complete electron transfer to the oxygen so that the electron returns to the iron atom when oxygen dissociates. 6. True/False Myoglobin has a greater affinity for oxygen than hemoglobin. Announcements • MLK Jr—No Discussion Next week • Discussion worksheets will be posted • Exam preparation – Review summaries – Review Notes – Homework Problems • Study Guide for more practice • Applications from Discussion Experiments in Protein Folding • Heat Denaturation • Renaturation – Often reversible – Primary sequence contains folding information RNase A • What happens to RNase A when – 2-mercaptoethanol is added? – When urea is added – When both are added? Chaotropic agent Scientists Draw Conclusions Protein Folding • Importance of Hydrophobicity • Cooperativity • Domains fold separately – Less than 200 aa is relatively fast Chaperones • Global energy minimum versus local energy minimum • Prevent misfolding or correct misfolding Case Study: Hemoglobin • • • • • Myoglobin Hemoglobin Heme Oxygen delivery Oxygen binding Structure of Myoglobin • Noncovalent binding • Hydrophobic pocket • Proximal and distal His Reversible Oxygen Binding Structure of Hemoglobin • Oligomer of four units resembling Mb • a2b2 tetramer • Treated as dimer of ab units Binding Constants and Curves • Simple mechanism is best! • From point of view of association: Protein + Molecule of Interest Complex Ka = [Complex]/[Free Protein][M] • Take home message: – Higher Ka means greater percentage bound • If Ka greater than 108, “tight binding” • If Ka less than 104, “little binding” – Applies to protein/protein interactions (4.9) Dissociation Constants • Assume excess of molecule of interest • KD = [P][M of I] [Bound P] Plot of % bound protein as a function of amount of molecule of interest Hyperbolic curve for linear scale Becomes sigmoidal for semi-log scale Apply to Myoglobin • Y = fraction bound (level of saturation) • Linear scale • Myoglobin is half saturated at 2.8 torr O2 • In tissue, myoglobin is nearly saturated Binding Curve of Hemoglobin • Hemoglobin is halfsaturated at 26 torr O2 • Hemoglobin has less affinity for oxygen • Hb saturated in lungs • When it reaches tissues, oxygen is released • Steep in important region • But why sigmoidal? Conformational Equilibrium • R T • Relaxed state binds oxygen well (low halfsaturation) • Tense state binds oxygen poorly • Equilibrium lies toward Tense state Hb Conformations • Binding of Oxygen changes shape of unit • Shape of subunit affects shapes of other subunits – Oxygen-bound unit causes other subunits to become relaxed – The rich become richer – Cooperative binding Observed Curve • At low [O2], curve looks like T • At high [O2], curve looks like R • The more oxygen that gets bound, the better more oxygen gets bound • Oxygen binding and offloading in the range of tissue oxygen Why is Tight Favored? • Allosteric regulation • 2,3-BPG • Holds all subunits toward the “Tight” conformation • No 2,3-BPG leads to halfsaturation at 12 torr • Physiological problem? Bohr Effect • pH also effects oxygen binding – Anaerobic exercise – High CO2 concentration • Lower pH leads to protonation of protein • Ion pairs form that stabilize the deoxy (unbound) form Answers 1. 2. 3. 4. 5. 6. E B A D T T