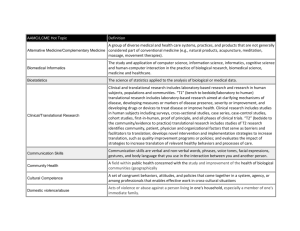
Standardization of phenotyping algorithms and data
advertisement
Session II: Standardization of
phenotyping algorithms and data
Jyotishman Pathak, PhD
Associate Professor of Biomedical Informatics
Department of Health Sciences Research
2015 AMIA Translational Summits, San Francisco
March 23rd, 2015
DISCLOSURES
Relevant Financial Relationship(s)
Mayo Clinic and Dr. Pathak have a financial
interest related to a device, product or
technology referenced in this presentation
with Apervita Inc.®
Off Label Usage
None
2015 AMIA Translational Summits
©2015 MFMER | slide-2
Outline
• Standards-based approaches to EHR
phenotyping
• NQF Quality Data Model
• JBoss® Drools business rules environment
• PhenotypePortal.org
• Standards-based approaches to phenotype
data representation
• Biomedical vocabularies and information models
• eleMAP data element harmonization toolkit
2015 AMIA Translational Summits
©2015 MFMER | slide-3
Key lessons learned from eMERGE
• Algorithm design and transportability
•
•
•
•
Non-trivial; requires significant expert involvement
Highly iterative process
Time-consuming manual chart reviews
Representation of “phenotype logic” is critical
• Standardized data access and representation
• Importance of unified vocabularies, data elements, and value sets
• Questionable reliability of ICD & CPT codes (e.g., billing the wrong
code since it is easier to find)
• Natural Language Processing (NLP) is critical
[Kho et al. Sc. Trans. Med 2011; 3(79): 1-7]
2015 AMIA Translational Summits
©2015 MFMER | slide-4
Example algorithm: Hypothyroidism
2015 AMIA Translational Summits
©2012 MFMER | slide-5
EHR-driven Phenotyping Algorithms
Rules
Evaluation
Phenotype
Algorithm
Transform
Mappings
Visualization
Transform
Data
NLP, SQL
2015 AMIA Translational Summits
[eMERGE Network]
©2015 MFMER | slide-6
Porting Phenotype Algorithms
• Initial eMERGE plan: sharing SQL, XML, near
executable pseudo-code
• That didn’t work: the sites were too heterogeneous
• More effective: sharing documents and humanreadable flowcharts
• Downside: requires humans to translate, each
implementation requires interpretation of ambiguity
• Moving forward: executable algorithm and
workflow specifications (back to the intentions of the
initial plan)
• Requirements: portability, standards-based (when
possible), buy-in at different levels
2015 AMIA Translational Summits
©2015 MFMER | slide-7
Algorithm Execution Process (baseline)
Human
Structured
Data
Extract &
Transform
Custom Scripts/Code
Text
NLP
Combine
Execute
Algorithm
Algorithm
Specifications
Custom
Scripts
Text
[eMERGE Network]
2015 AMIA Translational Summits
©2015 MFMER | slide-8
Pros and Cons
• Pros
• Very flexible
• “Portable” (with caveats)
• Cons
• Too flexible (no standard format)
• Requires implementation from scratch for every
•
algorithm
Error prone; can lead to ambiguity
2015 AMIA Translational Summits
©2015 MFMER | slide-9
Algorithm Execution Process
(standardized phenotype definitions)
Human
Structured
Data
Extract &
Transform
Custom Scripts/Code
Text
NLP
Combine
Execute
Algorithm
Algorithm
Specifications
Custom
Scripts
QDM
[eMERGE Network]
2015 AMIA Translational Summits
©2015 MFMER | slide-10
Pros and Cons
• Pros
• Consistent format
• Standards based
• Automatic translation to HTML (etc.) for human
consumption
• Cons
• Not as flexible as text
• May require extensions to cover algorithms not
•
expressible as Boolean rules
Still requires mapping to executable code
2015 AMIA Translational Summits
©2015 MFMER | slide-11
Algorithm Execution Process
(custom execution engine)
Automatic
Structured
Data
Extract &
Transform
Custom Scripts/Code
Text
Combine
NLP
Target
Execute
Algorithm
Algorithm
Specifications
Custom
Engine
QDM
[eMERGE Network]
2015 AMIA Translational Summits
©2015 MFMER | slide-12
Pros and Cons
• Pros
• Same as for previous, plus
• Consistent target for mapping specifications
• Allows possibility of automatic mapping
• Cons
• Need to transform source data into format
•
•
required by execution engine
Not portable to other institutions (may not be a
problem if you don’t care)
Unclear how to integrate NLP scripts/code
2015 AMIA Translational Summits
©2015 MFMER | slide-13
Algorithm Execution Process
(common execution engine)
Automatic
Structured
Data
Extract &
Transform
Custom Scripts/Code
Text
Combine
NLP
Target
Execute
Algorithm
Algorithm
Specifications
Drools,
KNIME
QDM
[eMERGE Network]
2015 AMIA Translational Summits
©2015 MFMER | slide-14
Pros and Cons
• Pros
• Same as for previous, plus
• Can re-use mappings developed externally
• Cons
• Need to transform source data into format
•
•
required by execution engine
Automatic mappings may be sub-optimal
Execution engine may not fit idiosyncratic
features of local data sources
2015 AMIA Translational Summits
©2015 MFMER | slide-15
NQF Quality Data Model (QDM) - I
• Standard of the National Quality Forum (NQF)
• A structure and grammar to represent quality measures
and phenotype definitions in a standardized format
• Groups of codes in a code set (ICD-9, etc.)
• "Diagnosis, Active: steroid induced diabetes" using
"steroid induced diabetes Value Set GROUPING
(2.16.840.1.113883.3.464.0001.113)”
• Supports temporality & sequences
• AND: "Procedure, Performed: eye exam" > 1 year(s)
starts before or during "Measurement end date"
• Implemented as a set of XML schemas
• Links to standardized terminologies (ICD-9, ICD-10,
SNOMED-CT, CPT-4, LOINC, RxNorm etc.)
2015 AMIA Translational Summits
©2015 MFMER | slide-16
NATIONAL QUALITY FORUM
NQF Quality Data Model (QDM) - II
[NQF QDM update, June 2012]
M Composition Diagram—The diagram depicts the definition of a QDM element beginning with defining a category, or the type of info
examples shown in the center blue boxes). The clear boxes on the left hand side of the drawing show the application of a state, or cont
2015 AMIA Translational Summits
©2015 MFMER | slide-17
NATIONAL
QUALITY
FORUM
NQF Quality
Data
Model
(QDM) - III
[NQF QDM update, June 2012]
Element Structure—The components of a QDM element are shown in the figure. The graphic on the left identifies the terms used
e QDM element. The graphic on the right uses each of these components to describe a QDM element indicating “Medication, admin
DM element is composed of a category, the state in which that category is expected to be used, and a value set of codes in a defined
2015 AMIA
Summits
©2015individual
MFMER attributes,
| slide-18
pecify which instance of the category is expected. The boxes
in theTranslational
lower section
of the QDM element specify
2015 AMIA Translational Summits
©2015 MFMER | slide-19
Example: Diabetes & Lipid Mgmt. - I
2015 AMIA Translational Summits
©2012 MFMER | slide-20
Example: Diabetes & Lipid Mgmt. - II
Human readable HTML
2015 AMIA Translational Summits
©2015 MFMER | slide-21
Example: Diabetes & Lipid Mgmt. - III
2015 AMIA Translational Summits
©2015 MFMER | slide-22
Example: Diabetes & Lipid Mgmt. - IV
2015 AMIA Translational Summits
©2015 MFMER | slide-23
Example: Diabetes & Lipid Mgmt. - V
Computer readable HQMF XML
(based on HL7 v3 RIM)
2015 AMIA Translational Summits
©2015 MFMER | slide-24
Modeling NQF criteria using Measure
Authoring Tool (MAT)
2015 AMIA Translational Summits
©2015 MFMER | slide-25
2015 AMIA Translational Summits
©2012 MFMER | slide-26
An Evaluation of the NQF Quality Data Model for Representing Electronic
Health Record Driven Phenotyping Algorithms
William K. Thompson, Ph.D.1, Luke V. Rasmussen1, Jennifer A. Pacheco1,
Peggy L. Peissig, M.B.A.2, Joshua C. Denny, M.D. 3, Abel N. Kho, M.D. 1,
Aaron Miller, Ph.D.2, Jyotishman Pathak, Ph.D.4,
1
Northwestern University, Chicago, IL; 2Marshfield Clinic, Marshfield, WI; 3Vanderbilt
University, Nashville, TN; 4Mayo Clinic, Rochester, MN
Abstract
The development of Electronic Health Record (EHR)-based phenotype selection algorithms is a non-trivial and
highly iterative process involving domain experts and informaticians. To make it easier to port algorithms across
institutions, it is desirable to represent them using an unambiguous formal specification language. For this purpose
we evaluated the recently developed National Quality Forum (NQF) information model designed for EHR-based
quality measures: the Quality Data Model (QDM). We selected 9 phenotyping algorithms that had been previously
developed as part of the eMERGE consortium and translated them into QDM format. Our study concluded that the
QDM contains several core elements that make it a promising format for EHR-driven phenotyping algorithms for
clinical research. However, we also found areas in which the QDM could be usefully extended, such as representing
information extracted from clinical text, and the ability to handle algorithms that do not consist of Boolean
combinations of criteria.
Introduction and Motivation
Identifying subjects for clinical trials and research studies can be a time-consuming and expensive process. For this
reason, there has been much interest in using the electronic health records (EHRs) to automatically identify patients
that match clinical study eligibility criteria, making it possible to leverage existing patient data to inexpensively and
automatically generate lists of patients that possess desired phenotypic traits.1,2 Yet the development of EHR-based
phenotyping algorithms is a non-trivial and highly iterative process involving domain experts and data analysts.3 It
is therefore desirable to make it as easy as possible to re-use such algorithms across institutions in order to minimize
the degree of effort involved, as well as the potential for errors due to ambiguity or under-specification. Part of the
solution to this issue is the adoption of an unambiguous and precise formal specification language for representing
phenotyping algorithms. This step is naturally a pre-condition for achieving the long-term goal of automatically
executable phenotyping algorithm specifications. A key element required to achieve this goal is a formal
representation for modeling the algorithms that can aid portability by enforcing standard syntax on algorithm
specifications, along with providing well-defined mappings to standard semantics of data elements and value sets.
[Thompson
et al., AMIA 2012]
Our experience in the development of phenotyping algorithms stems from work performed as part
of the electronic
4
Medical Records and Genomics (eMERGE) consortium, a network of seven sites originally using data collected in
the EHR as part of routine clinical care to
detect
phenotypes
for use
in genome-wide association studies. The
2015
AMIA
Translational
Summits
©2015 MFMER | slide-27
An Evaluation of the NQF Quality Data Model for Representing Electronic
Health Record Driven Phenotyping Algorithms
William K. Thompson, Ph.D.1, Luke V. Rasmussen1, Jennifer A. Pacheco1,
Peggy L. Peissig, M.B.A.2, Joshua C. Denny, M.D. 3, Abel N. Kho, M.D. 1,
Aaron Miller, Ph.D.2, Jyotishman Pathak, Ph.D.4,
1
Northwestern University, Chicago, IL; 2Marshfield Clinic, Marshfield, WI; 3Vanderbilt
University, Nashville, TN; 4Mayo Clinic, Rochester, MN
Abstract
The development of Electronic Health Record (EHR)-based phenotype selection algorithms is a non-trivial and
highly iterative process involving domain experts and informaticians. To make it easier to port algorithms across
institutions, it is desirable to represent them using an unambiguous formal specification language. For this purpose
we evaluated the recently developed National Quality Forum (NQF) information model designed for EHR-based
quality measures: the Quality Data Model (QDM). We selected 9 phenotyping algorithms that had been previously
developed as part of the eMERGE consortium and translated them into QDM format. Our study concluded that the
QDM contains several core elements that make it a promising format for EHR-driven phenotyping algorithms for
clinical research. However, we also found areas in which the QDM could be usefully extended, such as representing
information extracted from clinical text, and the ability to handle algorithms that do not consist of Boolean
combinations of criteria.
Introduction and Motivation
Identifying subjects for clinical trials and research studies can be a time-consuming and expensive process. For this
reason, there has been much interest in using the electronic health records (EHRs) to automatically identify patients
that match clinical study eligibility criteria, making it possible to leverage existing patient data to inexpensively and
automatically generate lists of patients that possess desired phenotypic traits.1,2 Yet the development of EHR-based
phenotyping algorithms is a non-trivial and highly iterative process involving domain experts and data analysts.3 It
is therefore desirable to make it as easy as possible to re-use such algorithms across institutions in order to minimize
the degree of effort involved, as well as the potential for errors due to ambiguity or under-specification. Part of the
solution to this issue is the adoption of an unambiguous and precise formal specification language for representing
phenotyping algorithms. This step is naturally a pre-condition for achieving the long-term goal of automatically
executable phenotyping algorithm specifications. A key element required to achieve this goal is a formal
representation for modeling the algorithms that can aid portability by enforcing standard syntax on algorithm
[Thompson
et al., AMIA 2012]
specifications, along with providing well-defined mappings to standard semantics of data elements
and value sets.
Our experience in the development of phenotyping algorithms stems from work performed as part of the electronic
Medical Records and Genomics (eMERGE)4 consortium, a network of seven sites originally using data collected in
the EHR as part of routine clinical care to
detect
phenotypes
for use
in genome-wide association
studies.
The | slide-28
2015
AMIA
Translational
Summits
©2015
MFMER
JBoss® Drools rules management system
• Represents knowledge with
declarative production rules
• Origins in artificial intelligence
expert systems
• Simple when <pattern> then
<action> rules specified in
text files
• Separation of data and logic
into separate components
• Forward chaining inference
model (Rete algorithm)
• Domain specific languages
(DSL)
2015 AMIA Translational Summits
©2015 MFMER | slide-29
Example Drools rule
{Rule Name}
rule
when
“Glucose <= 40, Insulin On”
{binding}
{Java Class}
{Class Getter Method}
$msg : GlucoseMsg(glucoseFinding <= 40,
currentInsulinDrip > 0 )
then
{Class Setter Method}
glucoseProtocolResult.setInstruction(GlucoseInstructions.
GLUCOSE_LESS_THAN_40_INSULIN_ON_MSG);
end
Parameter {Java Class}
2015 AMIA Translational Summits
©2015 MFMER | slide-30
Automatic translation from NQF criteria
to Drools
Measure
Authoring
Toolkit
From non-executable to
executable
Drools
Engine
Measures
XML-based
Structured
representation
Data Types
XML-based
structured
representation
Value Sets
Converting measures to
Drools scripts
Mapping data types
and value sets
Drools
scripts
Fact
Models
saved in XLS
files
2015 AMIA Translational Summits
©2015 MFMER | slide-31
[Li et al., AMIA 2012]
2015 AMIA Translational Summits
©2015 MFMER | slide-32
The “executable” Drools workflow
[Li et al., AMIA 2012]
2015 AMIA Translational Summits
©2015 MFMER | slide-33
http://phenotypeportal.org
[Endle et al., AMIA 2012]
1. Converts QDM to
Drools
2. Rule execution by
querying the CEM
database
3. Generate
summary reports
2015 AMIA Translational Summits
©2012 MFMER | slide-36
2015 AMIA Translational Summits
©2012 MFMER | slide-37
[Peterson AMIA 2014]
2015 AMIA Translational Summits
©2015 MFMER | slide-38
Example: Initial Patient Population criteria
for CMS eMeasure (CMS163V1)
2015 AMIA Translational Summits
©2012 MFMER | slide-39
[Peterson AMIA 2014]
2015 AMIA Translational Summits
©2015 MFMER | slide-40
Validation using Project Cypress
[Peterson AMIA 2014]
2015 AMIA Translational Summits
©2015 MFMER | slide-41
Alternative: Execution using KNIME
2015 AMIA Translational Summits
©2015 MFMER | slide-42
Outline
• Standards-based approaches to EHR
phenotyping
• NQF Quality Data Model
• JBoss® Drools business rules environment
• PhenotypePortal.org
• Standards-based approaches to phenotype
data representation
• Biomedical vocabularies and information models
• eleMAP data element harmonization toolkit
2015 AMIA Translational Summits
©2015 MFMER | slide-43
Overall Objective - I
• Without terminology and metadata standards:
• Health data is non-comparable
• Health systems cannot meaningfully
•
interoperate
Secondary uses of data for research and
applications (e.g., clinical decision support) is
not possible
• Our goal: Standardized and consistent
representation of eMERGE phenotype
data submitted to dbGaP (Database of
Genotypes and Phenotypes)
2015 AMIA Translational Summits
©2015 MFMER | slide-44
Overall Objective - II
1.Create/modify
a data
dictionary
2. Harmonize
data
elements to
standards
3. Generate
standardized
phenotype
data
2015 AMIA Translational Summits
Raw/Local Data from
EMR
©2015 MFMER | slide-45
What are Data Dictionaries?
• Data dictionaries
• Collections of variables
• Often constructed for a particular study
• May contain common and study-specific
variables
• Wide spectrum of formality
• Inconsistencies can complicate (or prevent) data
integration
• Differences in format (require transformations)
• Differences in semantic meaning
• Differences in data values
[PHONT RIPS 2011]
2015 AMIA Translational Summits
©2015 MFMER | slide-46
Data Element Standardization
Lab Measurements
trig_bl
Triglycerides (mg/dL)
Fasting triglycerides measured at Clinic
triglycerides Visit 1 in mg/dl units
Calculated variable: natural log of
tTriglycerides triglycerides
• Similar data element with different semantics
• Fasting vs. non-fasting (implied semantics?)
• Specific time is implied (visit number)
• Mathematically transformed (natural log)
[PHONT RIPS 2011]
2015 AMIA Translational Summits
©2015 MFMER | slide-47
Data Element Standardization
Medications
0 = No;
1 = Amiodarone (Cordarone,Pacerone);
2 = Sotalol (Betapace);
3 = Propafenone (Rhythmol);
4 = Quinidine (Quinaglute,Quinalan);
5 = Procainamide (Procanbid);
6 = Flecainide (Tambocor);
7 = Disopyramide (Norpace);
8 = Other;
9 = Mexilitene (Mexitil);
10 = Tikosyn (Dofetilide);
-8 = Not Applicable;
-1 = Unknown
• Different permissible values
1 = ACE Inhibitors;
2 = Amiodarone;
3 = Angiotensin Receptor Blockers;
4 = Beta Blockers;
5 = Calcium Channel Blockers;
6 = Class IA;
7 = Class IB;
8 = Class IC;
9 = Class III;
10 = Class V;
-1 = Unknown;
-1 = No Meds
• Can use standard drug ontologies
• Content and meaning of values
• Local meanings ("other",
"unknown")
• Could also have different
representations (codes vs. text)
(RxNorm)
• Standardize drug names and
classes (NDF-RT)
• Auto-classify agents (more
flexible, less error prone)
2015 AMIA Translational Summits
[PHONT RIPS 2011]
©2015 MFMER | slide-48
eMERGE Data Dictionary Standardization
• Data dictionary standardization effort
• Harmonize core eMERGE data elements
• Leverage standardized ontologies and metadata
• Develop SOPs for data dictionary usage
Standardization
Collect Dictionaries
Library of Standardized
Data Elements
[PHONT RIPS 2011]
2015 AMIA Translational Summits
©2015 MFMER | slide-49
Background: Clinical Terminology Standards
and Resources
• NCI Cancer Data Standards Repository
• Metadata registry based on ISO/IEC 11179 standard
•
•
•
for storing common data elements (CDEs)
Allows creating, editing, deploying, and finding of
CDEs
Provides the backbone for NCI’s semantic-computing
environment, including caBIG (Cancer Biomedical
Informatics Grid)
Approx. 40,000 CDEs
2015 AMIA Translational Summits
©2015 MFMER | slide-50
2015 AMIA Translational Summits
51
2015 AMIA Translational Summits
52
Background: Clinical Terminology Standards
and Resources
• CDISC Terminology
• To define and support terminology needs of the
•
•
CDISC models across the clinical trial continuum
Used as part of the Study Data Tabulation Model: an
international standard for clinical research data,
approved by the FDA as a standard electronic
submission format
Comprises approx. 2300 terms covering
demographics, interventions, findings, events, trial
design, units, frequency, and ECG terminology
2015 AMIA Translational Summits
©2015 MFMER | slide-53
2015 AMIA Translational Summits
54
Background: Clinical Terminology Standards
and Resources
• NCI Thesaurus
• Reference terminology for clinical care,
•
•
translational and basic cancer research
Comprises approx. 70,000 concepts
representing information for nearly 10,000
cancers and related diseases
NCI Enterprise Vocabulary Services
(LexEVS) provides the terminology
infrastructure for caBIG, NCBO etc.
2015 AMIA Translational Summits
©2015 MFMER | slide-55
2015 AMIA Translational Summits
56
2015 AMIA Translational Summits
57
Background: Clinical Terminology Standards
and Resources
• Systematized Nomenclature of Medicine Clinical
Terms is a comprehensive terminology covering
most areas of clinical information including
diseases, findings, procedures, microorganisms,
pharmaceuticals etc.
• Comprises approx. 370,000 concepts
• Acquired by International Health Terminology
Standards Development Organization (IHTSDO)
in 2007
2015 AMIA Translational Summits
©2015 MFMER | slide-58
2015 AMIA Translational Summits
59
Background: Clinical Terminology Standards
and Resources
• LOINC
• Logical Observation Identifiers Names and
•
•
Codes provides a set of universal codes and
names to identify laboratory and other clinical
observations
Over 100,000 terms
RELMA: Regenstreif LOINC Mapping
Assistant Program helps users map their
local terms or lab tests to universal LOINC
codes
2015 AMIA Translational Summits
©2015 MFMER | slide-60
2015 AMIA Translational Summits
61
eleMAP Conceptual Architecture
https://victr.vanderbilt.edu/e
MAP
2015 AMIA Translational Summits
©2015 MFMER | slide-62
eleMAP Data Harmonization Process
• 5 easy steps
1.Create a study
2.Create your data dictionary
3.Harmonize the data elements
4.Harmonize the actual/raw data
5.Iterate if necessary…
• Quick demo or Screen Shots
2015 AMIA Translational Summits
©2015 MFMER | slide-63
eleMAP Data Harmonization Process: 1
Step 1: Select “Harmonize Data” under My Account
2015 AMIA Translational Summits
©2015 MFMER | slide-64
eleMAP Data Harmonization Process: 2
Step 2: Select Study, Source, and Upload raw data file
2015 AMIA Translational Summits
©2015 MFMER | slide-65
eleMAP Data Harmonization Process: 3
Step 3: Click OK to confirm import of data file
2015 AMIA Translational Summits
©2015 MFMER | slide-66
eleMAP Data Harmonization Process: 4
Step 4: Display of harmonized data; Download file
2015 AMIA Translational Summits
©2015 MFMER | slide-67
eleMAP Data Harmonization Process: 5
Step 5: Harmonized data file for dbGaP submission
2015 AMIA Translational Summits
©2015 MFMER | slide-68
eleMAP Statistics (as of 03/09/2015)
• 18 different eMERGE studies
• 407 data elements, across 13 different
categories
•
•
•
•
•
68% mapped to caDSR CDEs
41% mapped to SNOMED CT concepts
41% mapped to NCI Thesaurus concepts
25% mapped to SDTM DEs
30% DEs have no mapping
2015 AMIA Translational Summits
©2015 MFMER | slide-69
Key lessons learned from eMERGE
phenotype data integration
Use case: eMERGE Network Combined Dataset
Studies: RBC, WBC, Height, Lipids, Diabetic Retinopathy, and
Hypothyroidism
1. Issue: Data value inconsistencies were found in common variables among
studies (e.g. race).
Suggestion: Use eleMAP to define study phenotypes/data elements and
disseminate finalized DD to all sites prior to actual data collection.
2. Issue: Same variable name was used for different data element concepts (e.g.
weight, height, and BMI)
Suggestion: Use eleMAP to review concept/description of existing data
elements and define new data elements if necessary.
3. Issue: Inconsistent data values were received (e.g. Sex). Some were original
values (F=Female; M=Male) and some were mapped values
(Female=C46110;Male=C46109).
Suggestion: Best to gather data in original values, combined data sets, and
then harmonize merged data files via eleMAP.
2015 AMIA Translational Summits
©2015 MFMER | slide-70
PheKB Data Dictionary Validation
• Validate data dictionary
• Columns
• Formatting
• Validate data file against data dictionary
• Variable names, order
• Data types, min, max
• Encoded values
2015 AMIA Translational Summits
©2015 MFMER | slide-71
PheKB Data Dictionary Validation
2015 AMIA Translational Summits
©2015 MFMER | slide-72
PheKB Data Dictionary Validation
2015 AMIA Translational Summits
©2015 MFMER | slide-73
PheKB Data Dictionary Validation
2015 AMIA Translational Summits
©2015 MFMER | slide-74
PheKB Data Dictionary Validation
2015 AMIA Translational Summits
©2015 MFMER | slide-75
Future eleMAP/PheKB Integration
• Tightly integrate data dictionary authoring in the
phenotype definition process
• Utilize eleMAP functionality to define the data
dictionary
• Submitted data sets validated against the builtin definition
2015 AMIA Translational Summits
©2015 MFMER | slide-76
Concluding remarks
• Standardization of phenotyping algorithms
and data dictionaries is critical
• Portability of algorithms across multiple
EMR environments
• Consistent and comparable data sets
• To the extent possible, the goal should be
to leverage on-going community-wide and
national standardization efforts
• Join the club!
2015 AMIA Translational Summits
©2015 MFMER | slide-77
Relevant presentations
• Monday (03/23/15)
• Session: TBI03 (Cyril Magnin III) @ 3:30PM
• Computational Phenotyping from Electronic
Health Records across National Networks
• Wednesday (03/25/15)
• Session: CRI02 (Embarcadero) @ 10:30AM
• A Modular Architecture for Electronic Health
Record-Driven Phenotyping
2015 AMIA Translational Summits
©2015 MFMER | slide-78
http://projectphema.org
2015 AMIA Translational Summits
©2015 MFMER | slide-79
Thank You!
Pathak.Jyotishman@mayo.edu
http://jyotishman.info
2015 AMIA Translational Summits
©2015 MFMER | slide-80