File - mrs. whalen's classes!
advertisement

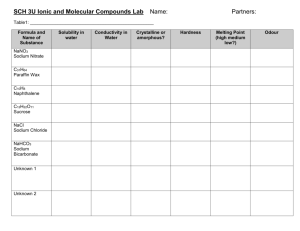
SCH3U Mrs. Whalen Physical Properties of Two Solids Purpose To determine the physical properties of two solids, one ionic (table salt, NaCl) and one covalent (camphor, C10H16O). Introduction Ionic and covalent bonds should be discussed. Include the types of elements involved, how the bond is formed (electron sharing, transfer), and how the bond types can be determined using electronegativity differences. Use Lewis structures in your introduction to demonstrate examples of each type of bonding. Discuss briefly the properties that will be tested in this lab – odour, melting point, hardness, solubility in water, and electrical conductivity in water. Provide a final statement that leads into the lab report. A good final statement summarizes for the reader what will be accomplished by the lab – read the Purpose of the lab, and restate it for your final statement. Question and Hypothesis To create a question for your lab, restate the Purpose in the form of an answerable question. Then, create a hypothesis. You are aware of the types of bonds in each of the two solids, and should have some knowledge of the properties to be expected (textbook and your own background knowledge). Discuss what you expect for each property – i.e. will each have an odour? A low or high melting point? Etc for each property. Observations Read through the procedure. Highlight any area that instructs you to make observations. Create an appropriate observations table. See format handout for instructions regarding naming your table. Procedure 1. Set up a retort stand with a ring clamp and wire gauze at a height that will be comfortable to observe. The cover for the ventilation system should be removed. Set up your Bunsen burner below the wire gauze. Do not light it yet. 2. Place a few crystals (10-20) of sodium chloride in your crucible. Observe the odour of the sodium chloride through wafting. Record your observations. 3. Place a small piece of camphor beside the sodium chloride in the crucible. Smell the camphor through wafting. Record your observations. 4. Place your crucible on your wire gauze. Light your Bunsen burner so that the flame is approximately an inch below the wire gauze. Heat your two samples until one begins to melt. Turn the Bunsen burner off as soon as melting has begun. Record your observations for melting point simply as high or low. Leave your samples to cool until you are done the lab. Do not handle while still hot. 5. Obtain two watch glasses. Put small samples of each solid on the same watch glass. Use the second watch glass to gently try to crush each sample. Record the hardness of each solid. 6. Set up two test tubes in a test tube rack. Place a small sample of NaCl in one and camphor in the other. Fill each test tube about half full with water. Mix the contents by carefully swirling each test tube. Record the solubility of each sample. Keep your test tubes for the next step. 7. Bring both test tubes to the front of the class. Pour a small amount of each into separate wells of a spot plate. Use a conductivity tester to determine the conductivity of each solid in water. Record your observations. 8. Pour any waste into the waste bucket at the front of the room. Rinse all materials into the waste bucket with a wash bottle. Clean all equipment thoroughly in the sink and return it to your lab cabinet. Discussion Answer the following questions in paragraph form. Be sure to reference your lab format handout! 1. Discuss the properties that were discovered for each of the two solids, relating them to the type of bonds found in each solid. Be sure to reference your observations during this discussion. 2. Which substance had the strongest odour? What does odour likely indicate about the ease with which the particles of a solid leave its surface? 3. Based on your observations of hardness and melting point, in which substance do you believe the molecules are most tightly bonded? Explain your reasoning. Extension Questions 1. Predict which compound is more soluble in water – I2 or CuCl2. Explain your prediction. 2. Predict which compound would have the lower melting point – Na2S or NO2. Explain your prediction.