Land and its resources TITLE INTRODUCTION
advertisement
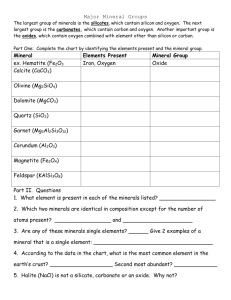
TITLE LAND AND ITS RESOURCES INTRODUCTION The Earth crust is a thin layer on the outer section of the Earth. There are various mineral in the earth crust. Look around you. Buildings, pencil lead, tap water, even the sand you step on, all come from mineral in the Earth crust. Minerals are much used in the building industry, transportation, industry, agriculture, and as natural fuel resources. WHAT YOU WILL LEARN ? Analyse the various minerals in the earth crust Understand the reactions between metal and non-metals Understand silicon compounds Analyse natural fuel resourses and their importance VARIOUS MINERALS IN THE EARTH CRUST Minerals are elements or chemical compounds are naturally present in the Earth crust. Examples of common minerals are gold, jade, limestone and tin ore. Minerals exist in the form of element or compound Examples of natural mineral elements are gold, silver(argentum), platinum and mercury Carbon and sulphur are two type of non-metal mineral element that exist naturally A FEW TYPES OF NATURAL MINERALS, MINERAL CONTENT AND ELEMENT THAT FORM IT natural minerals Type of ore Mineral content Element in the mineral Bauxite Aluminium ore Aluminium oxide Aluminium and oxygen Cassiterite Tin ore Tin oxide Tin and oxygen Hematite Iron ore Iron oxide Iron and oxygen Magnetite Magnesium oxide Magnesium carbonate Magnesium, carbon and oxygen Malachite Copper ore Copper carbonate Copper, carbon and oxygen Galena Lead ore Lead sulphide Lead and sulphur Iron pyrite Iron ore Iron sulphide Iron and sulphur GOLD JADE QUARTZ LIMESTONE CHARACTERISTICS OF MINERALS Most minerals are still in existence and have not changed in the Earth crust because minerals are generally hard and do not dissolve in water. Minerals like metal oxide and silicate normally do not decompose when heated . Some minerals like metal carbonates and sulphide can be easily decomposed by heating. The effect of heat decomposes the compound into its elements or other compounds. For example : heated metal carbonate metal oxide + carbon dioxide REACTION BETWEEN METALS AND NON-METALS A metal is an element with a surface that is shiny, ductile and can be forged. Examples of metal include zinc, magnesium, sodium, iron and many more. A non metal is an element with a surface that is dull and brittle Examples of non metal include graphite ( carbon ) , sulphur, oxygen and chlorine. REACTION OF METAL WITH OXYGEN Most metals react with oxygen to form metal oxide metal + oxygen metal oxide Example : Magnesium + oxygen Zinc + oxygen magnesium oxide zinc oxide Different metals have different reactivity with oxygen Metals like sodium and potassium are very active and easily combine with oxygen even though they may only be exposed to air. sodium + oxygen sodium oxide REACTION OF METALS WITH SULPHUR Most metals react with sulphur to form metal sulphide metal + sulphur metal sulphide Example : Iron + sulphur iron sulphide Zinc + sulphur zinc sulphide Just like the reaction between metal and oxygen, different metals have different reactivity with sulphur. SILICON COMPOUND Silicon is the second most abundant non-metals element found in the Earth crust. Silicon usually combines with other elements like metal and oxygen to from a silicon compound. Silica and silicate are silicon compound SILICA Silica is also known as silicon dioxide. Silica consists of a combination of silicon and oxygen elements in the following equation silicon + oxygen silicon dioxide(silica) Examples of silica are sand, quartz and flint. SILICATE Silicate is a silicon compound that contains silicon, metal and oxygen elements. Silicate is formed when silicon combines with oxygen and metal silicon + oxygen + metal silicate Examples of silicate include clay, mica, feldspar, and asbestos. Most ornamental stones like jade, ruby and topaz are silicate. CHARACTERISTIC OF SILICON COMPOUND Both silicon compound i.e silica and silicate, are very stable. Silicon compound (a) do not dissolve in water (b) do not react with acid (c) do not decompose when heated These characteristics of silicon compounds result in their existence as the second most abundant compound found in the Earth crust. NATURAL FUEL RESOURCES AND THEIR IMPORTANCE Natural fuel resouces found in the Earth crust include (a) petroleum (b) natural gas (c) coal (d) wood Petroleum and natural gas are hydrocarbon compounds because both consist of a compound mixture that is only formed from hydrogen and carbon elements. PETROLEUM Petroleum is a black and viscous liquid Petroleum is formed from decayed dead sea animals and plants that settled on the sea bed millions of years ago. Petroleum consists of mixture of various hydrocarbon compounds These various hydrocarbon compounds can be seperated through fractional distillation. This is because all the different types of hydrocarbon have different boling points. All petroleum fraction do not dissolve in water NATURAL GAS Natural gas is hydrocarbon compound usually obtained together with petroleum. Natural gas is used (a) as fuel in factoriesand in the home. (b) to make ammonia and nitrogen-based fertiliser COAL AND WOOD Coal is formed from swamp plants that died millions of years ago and have been compressed by layers of earth on top of it High pressure and temperature that act on these plants change them into coal Uses of coal include (a) generating electric energy in thermoelectric generating stations (b) warming the house in winter Uses of wood include (a) being turned into pulp to make paper (b) as planks used to make furniture, from the mangrove trees