Ledipasvir/sofosbuvir for 12 Weeks in Patients Coinfected With HCV
advertisement

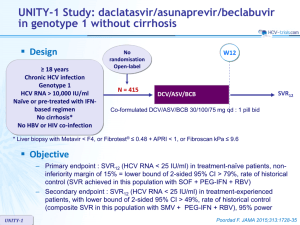
Ledipasvir/Sofosbuvir for 12 Weeks in Patients Coinfected With HCV and HIV-1: ION-4 Curtis Cooper1, Susanna Naggie2,Michael Saag3, Luisa M. Stamm4, Jenny C. Yang4, Phillip S. Pang4, John G. McHutchison4, Douglas Dieterich5, Mark Sulkowski6 1 University of Ottawa, 2Duke Clinical Research Institute, Durham, NC; The Ottawa Hospital, Ottawa, ON; of Alabama at Birmingham, Birmingham, AL,4Gilead Sciences, Inc., Foster City, CA; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6Johns Hopkins University School of Medicine, Baltimore, MD 3University IAS 2015, Vancouver Disclosures Dr. Cooper received funding as a : Speaker for Gilead Sciences, Abbvie Consultant for Gilead Sciences, Abbvie, MK, BMS Program Funding for Gilead Sciences, Abbvie. MK, Roche 2 Background Ledipasvir – Once-daily, oral, 90-mg NS5A inhibitor LDV NS5A inhibitor Sofosbuvir SOF nucleotide polymerase inhibitor ‒ Once-daily, oral, 400-mg NS5B inhibitor Ledipasvir/Sofosbuvir FDC – Once-daily, oral, fixed-dose (90/400 mg) combination tablet – Single-tablet regimen for hepatitis C FDC, fixed-dose combination. LDV NS5A LDV inhibitor NS5A inhibitor SOF SOF SOFnucleotide nucleotide SOF nucleotide polymerase polymerase nucleotide polymerase inhibitor inhibitor polymerase inhibitor inhibitor 3 Background and Aims HIV-HCV (ION-4) Liver-related complications remain a leading cause of death among HIV/HCV-coinfected patients1 Safe and effective oral treatments compatible with multiple antiretrovirals are needed for the eradication of HCV in HIV/HCV-coinfected patients Aim of this study was to evaluate the efficacy and safety of LDV/SOF for the treatment of HCV in patients coinfected with HIV-1, currently on antiretroviral therapy 1Smith, CJ et al. Lancet 2014; 384:241-8. 4 Study Design HIV-HCV (ION-4)) Wk 0 Wk 12 Wk 24 SVR12 N=335 LDV/SOF Phase 3, multicenter, open-label study (NCT02073656) HCV GT 1 or 4 patients in US, Canada, and New Zealand Broad inclusion criteria – HCV treatment-naïve or treatment-experienced – 20% with compensated cirrhosis – Platelets ≥50,000/mm3; hemoglobin ≥10 mg/dL, CrCl ≥60 mL/min – HIV-1 positive, HIV RNA <50 copies/mL; CD4 cell count >100 cells/mm3 ART regimens included emtricitabine and tenofovir disoproxil fumarate plus efavirenz, raltegravir, or rilpivirine 5 Endpoints HIV-HCV (ION-4) Primary efficacy endpoint: SVR12 – HCV RNA <LLOQ at post-treatment Week 12 HCV RNA analyzed by COBAS TaqMan HCV Test v2.0 HPS, with LLOQ of 25 IU/mL Safety – Adverse events and discontinuations – Maintenance of HIV-1 RNA <50 copies/mL – Serum creatinine 6 Results: Demographics and Baseline Characteristics HIV-HCV (ION-4) LDV/SOF 12 weeks N=335 Mean age, y (range) 52 (26-72) Male, n (%) 276 (82) Black, n (%) 115 (34) Hispanic or Latino, n (%) 56 (17) Mean BMI, kg/m2 (range) 27 (18-66) IL28B CC, n (%) 81 (24) GT 1 327 (98) HCV treatment experienced, n (%) 185 (55) Cirrhosis, n (%) 67 (20) Mean HCV RNA, log10 IU/mL ± SD Median CD4 cell count, cells/µL (range) 6.7 ± 0.6 628 (106-2069) HIV ARV Regimen Efavirenz + FTC + TDF 160 (48) Raltegravir + FTC + TDF 146 (44) Rilpivirine + FTC + TDF 29 (9) 7 Results: SVR12 HIV-HCV (ION-4) Overall 100 Naïve vs Experienced Cirrhosis Status 96 95 97 96 94 321/335 142/150 179/185 258/268 63/67 Naïve Experienced No Cirrhosis Cirrhosis SVR12 (%) 80 60 40 20 0 LDV/SOF 12 Weeks Error bars represent 95% confidence intervals. 8 Results: SVR12 HIV-HCV (ION-4) Overall Overall 100 96 Naïve vs Experienced 95 97 Cirrhosis Status 96 94 SVR12 (%) 80 60 40 • • • • 10 relapses 2 on-treatment failures (noncompliance, per investigators) 1 lost to follow-up 1 death (IVDU-related endocarditis/sepsis) 20 321/335 142/150 179/185 258/268 63/67 Naïve Experienced No Cirrhosis Cirrhosis 0 LDV/SOF 12 Weeks Error bars represent 95% confidence intervals. 9 Results: SVR12 by Prior Treatment Experience HIV-HCV (ION-4) Overall Overall 100 Naïve vs Experienced Cirrhosis Status 96 95 97 96 94 321/335 142/150 179/185 258/268 63/67 Naïve Experienced No Cirrhosis Cirrhosis SVR12 (%) 80 60 40 20 0 LDV/SOF 12 Weeks Error bars represent 95% confidence intervals. 10 Results: SVR12 by Prior Treatment Experience and Cirrhosis Status HIV-HCV (ION-4) Overall Overall 100 Naïve vs Experienced Cirrhosis Status 96 95 97 96 94 321/335 142/150 179/185 258/268 63/67 Naïve Experienced No Cirrhosis Cirrhosis SVR12 (%) 80 60 40 20 0 LDV/SOF 12 Weeks Error bars represent 95% confidence intervals. 11 Results: SVR12 in Subgroups HIV-HCV (ION-4) LDV/SOF 12 Weeks, N=335 Overall Sex Race HCV Genotype Baseline HCV RNA (IU/mL) Baseline BMI (kg/m2) IL28B Cirrhosis Prior HCV Treatment ARV Regimen Baseline CD4 (cells/μL) Male Female Black Non-Black 1a 1b 4 <800,000 ≥800,000 <30 ≥30 CC CT TT No Yes No Yes EFV + FTC + TDF RAL + FTC + TDF RPV + FTC + TDF <350 ≥350 60 70 80 90 SVR12, % (95% CI) 100 12 Results: SVR12 in Subgroups HIV-HCV (ION-4) LDV/SOF 12 Weeks, N=335 Overall Sex Race HCV Genotype Baseline HCV RNA (IU/mL) Baseline BMI (kg/m2) IL28B Cirrhosis Prior HCV Treatment ARV Regimen Baseline CD4 (cells/μL) Male Female Black Non-Black 1a 1b 4 <800,000 ≥800,000 <30 ≥30 CC CT TT No Yes No Yes EFV + FTC + TDF RAL + FTC + TDF RPV + FTC + TDF <350 ≥350 60 Statistically significant in multivariate analysis 70 80 90 SVR12, % (95% CI) 100 13 ION-4 – LDV/SOF in HIV/HCV SVR12 by ARV Regimen and Race Non-Black 100 100 99 90 Black 98 100 95 90 85 % SVR12 80 60 40 20 215/217 103/115 97/97 52/61 13/13 100/102 42/44 49/50 18/18 9/10 0 Overall EFV/FTC/TDF RAL+FTC/TDF RPV/FTC/TDF PK and Other Exploratory Analyses HIV-HCV (ION-4) No difference in SVR in HCV mono-infected ION program (12 weeks) for black (89/90, 99%) versus non-black (431/448, 96%)2 LDV and SOF population PK levels – Similar across the different ARV regimens – Similar between black and non-black patients – Similar between patients who relapsed and those who achieved SVR GWAS and whole genome sequencing analysis underway 2Lennox et al. AASLD 2014 Oral abstract #237 15 Results: HCV Sequence Analysis HIV-HCV (ION-4) Deep sequencing of NS5A at baseline identified 59 (18%) patients with NS5A variants (RAVs) – 55 (93%) of patients with NS5A RAVs achieved SVR12 Post-treatment NS5A RAVs were observed in 10 of the 12 patients with virologic failure No NS5B S282T was observed in any patient at baseline or virologic failure 16 Results: Safety Summary HIV-HCV (ION-4) Patients, n (%) AEs Overall safety LDV/SOF 12 Weeks N=335 257 (77) Grade 3‒4 AE 14 (4) Serious AE 8 (2)* Treatment D/C due to AE 0 Death 1 (<1)† Grade 3‒4 laboratory abnormality 36 (11) Stable CD4 counts through treatment and follow-up phase No patient had confirmed HIV virologic rebound *Serious AEs in >1 patient were hepatocellular carcinoma (n=2) and portal vein thrombosis (n=2) in patients with cirrhosis. †Confirmed IV drug user developed Staphylococcus aureus sepsis, endocarditis with associated embolic brain abscesses, and multi-organ system failure. 17 Results: Adverse Events (≥5%) HIV-HCV (ION-4) Patients, n (%) LDV/SOF 12 Weeks N=335 Headache 83 (25) Fatigue 71 (21) Diarrhea 36 (11) Nausea 33 (10) Arthralgia 22 (7) Upper respiratory tract infection 18 (5) 18 Results: Renal Function HIV-HCV (ION-4) Creatinine Clearance (mL/min), mean ± SD LDV/SOF + EFV+FTC+TDF (n=160) 150 RAL+FTC+TDF (n=146) 140 RPV+FTC+TDF (n=29) 130 120 110 100 90 80 70 60 BL 1 2 4 6 8 10 12 FU-4 Week 4 patients (1%) had change in creatinine ≥ 0.4 mg/dL – 2 completed treatment with no ART change – 1 had dose reduction of TDF, 1 discontinued TDF 19 Conclusions HIV-HCV (ION-4) In this Phase 3 study of 335 HIV/HCV-coinfected patients, 96% achieved SVR12 after 12 weeks of a once-daily, single-tablet regimen of LDV/SOF – Prior HCV treatment status or the presence or absence of cirrhosis did not impact outcome – In contrast to larger studies among monoinfected patients, a lower response rate was observed among coinfected black patients treated with LDV/SOF (SVR12 90%) LDV/SOF was well tolerated, with no treatment discontinuations due to adverse events and no adverse impact on HIV disease or its treatment 20 Acknowledgments We extend our thanks to: The patients and their families All participating investigators throughout the US, Canada, and New Zealand UNITED STATES: David Asmuth, Rachel Baden, Meena Bansal, Maurizio Bonacini, Norbert Brau, U. Fritz Bredeek, Raymond Chung, Calvin J. Cohen, Eric Daar, Craig Dietz, Robin Henry Dretler, Richard Elion, W.J. Fessel, Jason Flamm, Timonthy Friel, Joel E. Gallant, Joseph C. Gathe, Eliot Godofsky, Philip M. Grant, Federico Hinestrosa, Gregory Huhn, Mamta Jain, Dushyantha Jayaweera, Donald Kotler, Jay Lalezari, Charles Landis, Annie Luetkemeyer, Kristen Marks, Cynthia Mayer, Anthony Mills, Karam Mounzer, Susanna Naggie, Bruce S. Rashbaum, Jorge E. Rodriguez, Peter J. Ruane, Paul Edward Sax, Michael Saag, Kenneth Sherman, Marc Siegel, Richard Sterling, Mark Sulkowski, Karen T. Tashima, Pablo Tebas, Melanie A. Thompson, William J. Towner, Chia Wang, David A. Wheeler, David A. Wohl, Kimberly Workowski, David Wyles, Benjamin Young CANADA: Curtis Cooper, Emmanuelle Huchet, Mark Hull, Marina Klein, David Wong PUERTO RICO: Javier O. Morales-Ramirez, Jorge L. Santana-Bagur NEW ZEALAND: Edward Gane, Catherine Stedman This study was funded by Gilead Sciences, Inc. 22