Hydrocarbons
advertisement
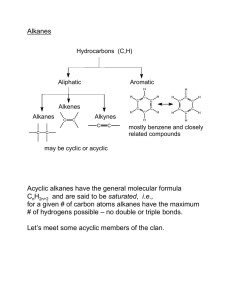
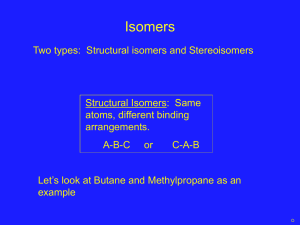
Hydrocarbons • A hydrocarbon is a compound containing the elements H and C only. • A Homologous Series is a group of Hydrocarbons with the same General Formula and similar Chemical Properties. The Alkanes • Simplest group of Hydrocarbons. • General formula – CnH2n+2( n= number of Carbon atoms) • Mainly used as fuels. • Have only Carbon to Carbon single bonds in their structure. • This makes them SATURATED. Alkanes – more info! • • • • • • • • • Name Methane Ethane Propane Butane Pentane Hexane Heptane Octane Molecular Formula CH4 C2H6 C3H8 C4H10 C5H12 C6H14 C7H16 C8H18 Alkane - Structure Alkenes • • • • • General Formula CnH2n Contain C=C double bonds. They are Unsaturated. Again can be used as fuels. Test – decolourise Bromine water – INSTANTLY! • Colour change Brown - Clear The Names! • Alkene Ethene Propene Butene Pentene Hexene Heptene Octene Molecular formula C2H4 C3H6 C4H8 C5H10 C6H12 C7H14 C8H16 Alkene - Structure Cycloalkanes • General formula CnH2n • They are saturated – no double bonds. • They have a ring in their structure. • They are isomers with alkenes. Cycloalkane - names • • • • • • • Name Molecular Formula Cyclopropane C3H6 Cyclobutane C4H8 Cyclopentane C5H10 Cyclohexane C6H12 Cycloheptane C7H14 Cyclooctane C8H16 Cycloalkane - Structure Cracking! • There are more of the heavier less useful fractions. • We can “chop” them up into smaller – more useful ones. • This is called “Cracking” • We need heat and a catalyst for this! Products of Cracking • An Unsaturated product (contains double bond) and a Saturated product ( single bonds only) are always formed. • Example • C12H26 —>C4H8 + C6H14 +C2H4 Isomers • Isomers are compounds with the same molecular formula but a different structural formula. • Example • C4H10 – this has 2 isomers. • H H H H H CH3 H I I I I I I I • H - C – C – C – C – H and H - C – C – C - H I I I I I I I H H H H H H H More Isomers • The alkenes and cyclo alkanes are isomers. • Example • C3H6 is the molecular formula for both propene and cyclo propane. • They both have different structures.