Unit 2 Study Guide - Madison County Schools
advertisement

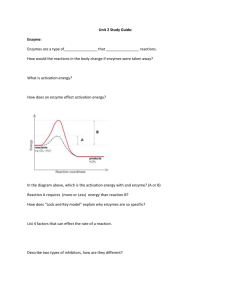
Unit 2 Study Guide KEY: Enzyme: Enzymes are a type of ____catalyst___ that ____speeds up_____ reactions. How would the reactions in the body change if enzymes were taken away? they would slow down so much that life would be unable to exist. What is activation energy? the energy required to start a reaction. How does an enzyme effect activation energy? It lowers it. In the diagram above, which is the activation energy with and enzyme? (A or B) Reaction A requires (more or Less) energy than reaction B? How does “Lock and Key model” explain why enzymes are so specific? Because an enzyme will only bond to a specific substrate, just like a lock will only work with a specific key. List 4 factors that can effect the rate of a reaction. substrate concentration, enzyme concentration, pH, temperature, inhibitors Describe two types of inhibitors, how are they different? competitive inhibitor binds at the active site, blocking the substrate non-competitive inhibitor binds somewhere other than the active site, changing the shape of the active site so that the substrate no longer fits. Explain what this diagram above is showing. That as substrate concentration increases, the rate of the reaction oncreases. But the reaction will level off (stop icreasing in rate) after so much substrate is added. r How is an enzyme affected by being outside its “range”(such as pH or temperature range)? It will denature. What does it mean when an enzyme is denatured? It will lose its shape, as well as its function. Acids / Bases: Draw and label the range of the pH scale, label the acidic, neutral, and basic regions? <------------------------------------------------------------> 1 7 14 acid neutral base Water: What is surface tension, and what makes it possible? The ability for water to form a "film" on it's surface. Hydrogen bonds between water molecules link them all together, forming a type of "net" on water's surface. How does soap effect surface tension? Soap will break the hydrogen bonds that hold the water molecules together, thus destroying surface tension. What is cohesion? attraction between like substances (water molecules are attracted to water molecules) What is adhesion? attraction between unlike substances (water molecules are attracted to glass) What is capillary action? A plants ability to draw water up its root system, even against gravity. Organic compounds: What element do ALL organic compounds contain? Carbon List 2 examples of monosaccharides, and where they are found in life. Glucose - found in your blood. Fructose - found in fruit. List 2 examples of disaccharides, and where they are found in life. Sucrose - table sugar. Lactose - milk sugar. List 3 examples of polysaccharides, and where they are found in life. Starch - plants (for energy storage). Cellulose - plants (for structure). Glycogen - animals (energy storage) Draw the structures of the following 2 monosaccharides, 1 disaccharide. glucose: fructose: Which polysaccharide is un-digestible by humans? cellulose Proteins: What are the building blocks of proteins? Amino Acids Name an 3 examples of a protein's function in life. structure (keratin, collagen) Enzymes (lactase, amylase) Transport (hemoglobin) Defense (antibodies) Hormones (insulin) Sucrose: Lactose: Lipids: List 2 examples of lipids, and explain their function in life. phospholipids (build cell membranes) steroids (testosterone, estrogen) Fats (energy storage) waxes (protection, waterproofing) What parts make up a lipid (triglyceride)? glycerol head, and three fatty acid tails What are the 3 main difference between a saturated fat and an unsaturated fat? Unsaturated have double bonds, are kinked, and are liquid. Saturated have no double bonds, are straight, and are solid. Which one is saturated: Nucleic Acids: What are the building blocks of nucleic acids? nucleotides Name 2 examples of nucleic acids, and explain their function. DNA, and RNA (information storage)