RESPIRATORY SYSTEM
advertisement

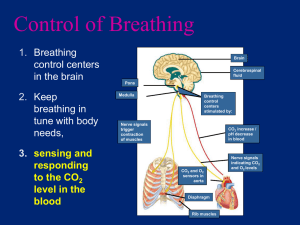
RESPIRATORY SYSTEM Marieb - Chapter 22 Respiratory Processes •Pulmonary Ventilation •External Respiration •Transport of Respiratory Gases •Internal Respiration Conducting Zone Respiratory Zone Larynx Cartilages of the Larynx Trachea & Bronchi Trachea Bronchi & Bronchioles Bronchioles and Alveoli Bronchiole if <1mm diameter Boyle’s Law P1V1 = P2V2 Robert Boyle (1627-1691) Physical Factors Influencing Pulmonary Ventilation • Airway Resistance - The major source is friction encountered in the respiratory passages. • Alveolar Surface Tension - force created by alveolar fluid that resists lung distension. Surfactant (surface active agent) lowers surface tension. • Lung Compliance - The distensibility (stretchiness) of lung tissue and the thoracic cage. ASTHMA • Bronchiole smooth muscle is sensitive to neural and chemical controls. • In an asthma attack, bronchioles first become inflamed, then the muscle constricts causing airways to narrow. • Risk factors include exposure to indoor allergens during infancy, allergies, and a family history of asthma. Respiratory Volumes M F Minute Ventilation = Tidal Volume X Respiratory Rate MV = TV x RR = 500 ml/breath X 12 breaths/min = 6000 ml/min Dead volume = 1ml/pound ideal body weight “Fresh” inspired air is diluted by the left over air remaining in the lungs from the previous breathing cycle. Alveolar Ventilation = (Tidal Volume - Dead Space) X Respiratory Rate AVR = (TV - ADS) x RR = (500 ml/breath - 150 ml) X 12 breaths/min = 350 ml/breath X 12 breaths/min = 4200 ml/min Gaseous Exchange Dalton’s Law of Partial Pressures Ptotal = P1 + P2 + . . . Pn John Dalton (1766-1844) Gaseous Exchange Henry’s Law A gas will dissolve in a liquid in proportion to its partial pressure William Henry (1775-1836) N2 78.6 597 74.9 569 O2 20.9 159 13.7 104 CO2 0.04 0.3 5.2 40 H2O 0.46 3.7 6.2 47 100% 760 100% 760 1 atmosphere= 1 Torr = 760mmHg N2 78.6 597 74.9 569 O2 20.9 159 13.7 104 CO2 0.04 0.3 5.2 40 H2O 0.46 3.7 6.2 47 100% 760 100% 760 Gas Solubilities • CO2 is about 20X more soluble in plasma than O2 • N2 is practically insoluble. Respiratory Membrane 5 m thick in healthy lungs thickness gas diffusion Total Surface Area 75 m2 in healthy lungs surface area gas diffusion OXYGEN TRANSPORT 1.5% dissolved in plasma 98.5% bound to hemoglobin HHb + O2 Lungs tissues HbO2 + H+ O2 Dissociation Curve PO2 lung = 104mmHg PO2 Bohr Effect CARBON DIOXIDE TRANSPORT 7-10% dissolved in plasma ~20% bound to hemoglobin CO2 + Hb HbCO2 ~70% as bicarbonate ion (HCO3-) CO2 + H2O carbonic anhydrase H2CO3 H+ + HCO3- CO2 Transport: Tissue Figure 22.22a • CO2 diffuses into plasma and into the RBC – RBCs contain carbonic anhydrase which rapidly combine H2O and CO2 to form H2CO3 – H2CO3 quickly dissociates to H+ and HCO3– H+ ions become associated with protein portion of Hb (triggers the Bohr effect) – HCO3- rapidly diffuses out of RBC into plasma – Cl- moves into RBC to balance ionic charges (chloride shift) CO2 Transport: Lung Figure 22.22b • In the lungs the process reverses – – – – – HCO3- moves into the RBC Cl- shifts out of RBC HCO3- binds H+ g H2CO3 Carbonic anhydrase splits H2CO3 g H2O + CO2 CO2 moves down its partial pressure gradient to the alveoli