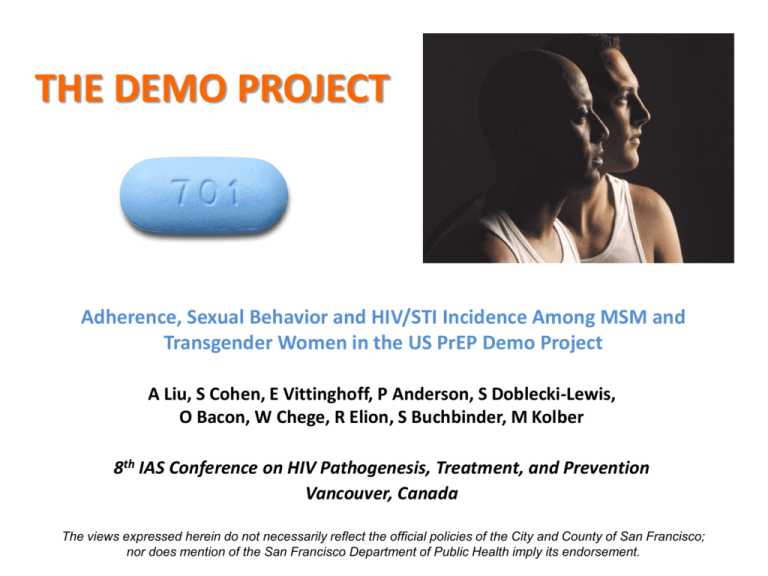
THE DEMO PROJECT
Adherence, Sexual Behavior and HIV/STI Incidence Among MSM and
Transgender Women in the US PrEP Demo Project
A Liu, S Cohen, E Vittinghoff, P Anderson, S Doblecki-Lewis,
O Bacon, W Chege, R Elion, S Buchbinder, M Kolber
8th IAS Conference on HIV Pathogenesis, Treatment, and Prevention
Vancouver, Canada
The views expressed herein do not necessarily reflect the official policies of the City and County of San Francisco;
nor does mention of the San Francisco Department of Public Health imply its endorsement.
Disclosures
• I have received research funding for PrEP from the
US NIH and support for manuscript writing from IASUSA.
• For several research studies, including this project,
Gilead Sciences provided study drug and supported
drug level testing for this project.
?
Background
• Several RCTs have shown that daily oral TDF/FTC is
effective in preventing HIV infection1-4
• Little known about PrEP implementation outside of
clinical trials
– Concerns include poor adherence, risk compensation, drug resistance,
and safety/toxicity
• Demonstration projects recommended to address implementation
issues and determine how best to scale up PrEP5
• STD and community-based clinics serving populations at risk are
promising clinical sites for PrEP delivery in the US
1Grant NEJM
2010; 2Baeten NEJM 2012; 3Thigpen NEJM 2012; 4Choopanya Lancet 2013
5WHO Guidance on Oral Pre-Exposure Prophylaxis July 2012
The Demo Project
• Multisite, open-label PrEP
Demonstration Project in MSM and
transgender women in STD clinics and
a community health center in the US
• Key objectives:
– PrEP uptake (high interest and uptake
previously published1)
– Adherence
– Sexual behaviors
– STI/HIV incidence
– HIV resistance, safety
1Cohen S
CROI 2014, JAIDS 2015
PrEP
Demo Project Sites
San Francisco City Clinic
(N=300)
Miami-Dade County
Downtown STD clinic (N=157)
Whitman Walker Health
(N=100)
• Annual HIV seroconversion rate among MSM >2% across clinics
• Participants were either clinic referred (46%) or self-referred (54%)
Methods
• HIV-negative MSM and transgender women enrolled
between Oct 2012 - Jan 2014
• Behavioral risk criteria (last 12 mo):
– Condomless anal sex with 2+ partners
– 2+ episodes of anal sex with HIV+ partner
– Syphilis, rectal gonorrhea or chlamydia diagnosis
• No serious medical conditions: CrCl ≥ 60 ml/min,
negative/trace protein on urine dipstick, HbSAg negative
• Participants offered up to 48 weeks of TDF/FTC PrEP
• Follow-up at 1, 3, 6, 9, and 12 months for HIV/STI testing,
counseling, clinical monitoring, PrEP dispensation
Methods: (cont’d)
• PrEP adherence (all visits)
– Self-reported adherence rating scale
– Medication possession ratio (pills dispensed/total days between visits)
– Dried blood spots for tenofovir diphosphate (TFV-DP)
• Random sample of ~100 participants/site tested*
• Protective TFV-DP levels associated with ≥700 fmol/punch (≥4 doses/week)1,2
• PrEP engagement: 5-level ordinal measure
TFV-DP (fmol/punch)
Adherence Interpretation
700
≥4 doses/week
350 to 699
2-3 doses/week
<350
< 2 doses/week
BLQ
No recent dosing
Missed visit
Missed visit
• Sexual and drug use behaviors: quarterly interview
• Adherence & sexual/drug behaviors evaluated using GEE & Poisson models
1Grant Lancet
ID 2014; 2Castillo-Mancilla et al. AIDS Res Hum Retroviruses 2013
*DBS from all Black and transgender ppts tested; results weighted to reflect overall cohort
Baseline characteristics of enrolled participants
Characteristic
%
Age (median)
35 years
with 20% <25 years
Race/ethnicity
White
Latino
Black
Other
48%
35%
7%
10%
Gender
Male
Transgender
98%
1.3%
Education level
≤ High School
Some college or higher
15%
85%
Any recreational drug use
Popper, cocaine, meth, or club drug use
74%
58%
Number of anal sex partners, past 3 months (mean)
11
Condomless receptive anal sex, past 3 mo
67%
HIV+ primary partner
24%
STI (GC, CT, syphilis) at baseline
26%
Results: Retention
• Total of 481 person years of follow-up
• Retention at visits: 93%, 88%, 85%, 80%, 78%
at 1, 3, 6, 9, and 12 months respectively
• Retention higher in those with prior PrEP
knowledge and reporting condomless receptive
anal sex at baseline; lower in Miami
Results: Adherence
100%
Protective TFV-DP in
DBS
90%
Rating scale: very
good/excellent
80%
70%
Medication Possession
Ratio (mean)
Percent
60%
•
50%
40%
•
30%
•
20%
10%
0%
4
12
24
Visit week
36
48
63% had protective
DBS levels at all visits
3% always had DBS
levels <2 doses/week
PrEP dispensation
interrupted in 15%:
most commonly due
to side effect
concerns or low
perceived risk
Independent predictors of protective DBS levels
Characteristic
% PL*
AOR (95% CI)
P value
Site
San Francisco
Miami
DC
90
65
88
Ref
0.32 (0.17-0.60)
1.08 (0.54-2.19)
<0.001
0.82
Race/Ethnicity
White
Latino
Black
Asian
Other
91
77
57
84
82
Ref
0.81 (0.41-1.61)
0.28 (0.12-0.64)
0.72 (0.17-3.03)
0.42 (0.13-1.38)
0.55
0.003
0.65
0.15
Living situation
Rent or own housing
Other
87
70
2.02 (1.14-3.55)
Reference
# condomless anal sex partners, past 3 mo
0-1
≥2
75
89
Reference
1.82 (1.14-2.89)
*PL = Protective DBS levels (TFV-DP in DBS consistent with ≥4 doses/week)
OR for protective levels did not differ by age, education, alcohol, or drug use
0.02
0.01
Results: PrEP Engagement
Lower Engagement in Miami and among African-American participants
Results: Sexual behaviors
Mean number of anal sex partners declined from 10.9 to 9.3 (p=0.04)
70
12
60
10
% reporting ncRAI
8
40
6
30
4
20
10
2
0
0
Screening
12
24
36
Visit week
48
Mean Number of RAI episodes
50
Reported ncRAI (%)
Mean RAI episodes with a
condom
Mean RAI episodes without
a condom
Results: STI positivity
18
STI positivity rate at each visit (%)
16
14
12
Rectal STI (GC/CT)
10
Urethral STI (GC/CT)
8
Pharyngeal STI (GC/CT)
6
Primary, secondary, or early
latent syphilis
4
2
0
Screening
12
24
36
48
Visit interval
Overall STI incidence (90/100 person years) remained stable during follow-up (P>0.1)
Results: HIV seroconversions and incidence
• 3 acute infections at enrollment
– All had negative rapid and 4th gen HIV tests
– 2 had positive pooled RNA, 1 positive individual RNA
– FTC resistance (M184V) developed in one ppt 1 week after
enrollment: suppressed on combination ART
• Only 2 infections during follow-up
– PPT #1: 19 weeks after enrollment:
Reported last dose >1 month prior, TFV-DP levels < 2 doses/wk
– PPT #2: 4 weeks after 48 week visit:
TFV-DP levels < 2 doses/wk or undetectable after week 4.
– No evidence of HIV resistance
• HIV incidence = 0.43 / 100 py (95% CI 0.05-1.54)
Safety
• 19 serious adverse events: none assessed as
related to TDF/FTC
• 23 creatinine elevations in 13 (2.3%) ppts
– All grade 1, except one grade 2
– Only 3 confirmed on repeat testing, all resolved
without stopping PrEP
• 12 bone fractures reported during the study:
all but one (tooth fracture) explained by
trauma; none assessed as related to TDF/FTC
Limitations
• African-American and transgender persons underrepresented
– Reflects under-representation at participating clinics
– Highlights the need for increased outreach and
engagement in these communities around PrEP
• Results may not generalize to broader MSM
populations in these or other US cities, international
settings
• Demo Project provide free PrEP medication and
monitoring -- cost and lack of insurance coverage
may reduce PrEP access and adherence
Conclusions
• PrEP adherence relatively high among MSM receiving PrEP in
STD and community health center in the Demo Project
– Higher adherence among those at higher risk → increased costeffectiveness and impact of PrEP1,2
• HIV incidence extremely low, despite high incidence of STIs:
provides strong support for the scale-up of PrEP in these
clinical settings
• Quarterly STI screening, including testing at extragenital sites,
recommended for MSM taking PrEP
• Interventions to address racial and geographic disparities and
housing instability may increase PrEP impact in the US
1Juusola Ann
Intern Med 2012; 2Kessler AIDS 2014
Acknowledgements
San Francisco
Stephanie Cohen
Susan Buchbinder
Oliver Bacon
Robert Blue
Nikole Trainor
Susan Philip
Tim Matheson
Erin Andrew
Miami
Michael Kolber
Susanne Doblecki-Lewis
Jose Castro
Yannine Estrada
Daniel Feaster
Gabriel Cardenas
DC
Rick Elion
Megan Coleman
Justin Schmandt
Other Co-Investigators
Eric Vittinghoff
Peter Anderson
Teri Liegler
K. Rivet Amico
Robert Grant
NIAID
Wairimu Chege
Cherlynn Mathias
David Burns
Michelle Wildman
NIMH
Michael Stirratt
Chris Gordon
DF/Net
Brian Postle
Gilead
Jim Rooney
Keith Rawlings
Study participants