What is an acid?
advertisement
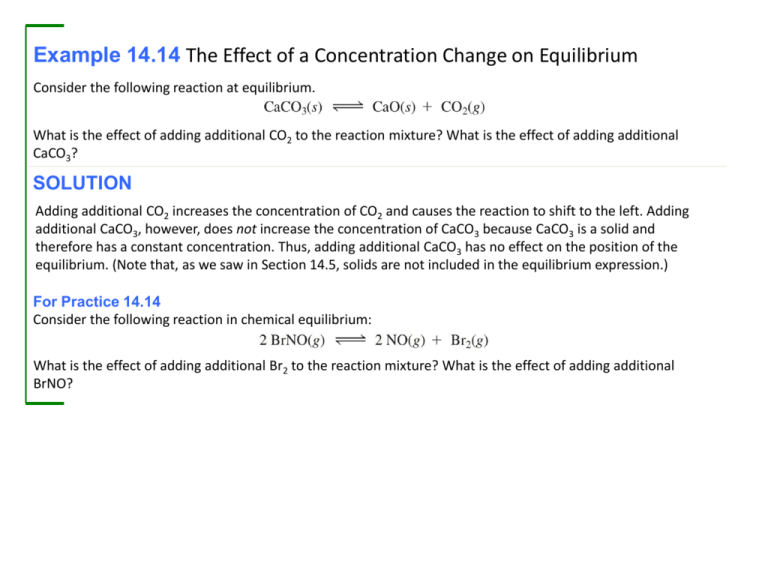
Example 14.14 The Effect of a Concentration Change on Equilibrium Consider the following reaction at equilibrium. What is the effect of adding additional CO2 to the reaction mixture? What is the effect of adding additional CaCO3? SOLUTION Adding additional CO2 increases the concentration of CO2 and causes the reaction to shift to the left. Adding additional CaCO3, however, does not increase the concentration of CaCO3 because CaCO3 is a solid and therefore has a constant concentration. Thus, adding additional CaCO3 has no effect on the position of the equilibrium. (Note that, as we saw in Section 14.5, solids are not included in the equilibrium expression.) For Practice 14.14 Consider the following reaction in chemical equilibrium: What is the effect of adding additional Br2 to the reaction mixture? What is the effect of adding additional BrNO? Example 14.15 The Effect of a Volume Change on Equilibrium Consider the following reaction at chemical equilibrium: What is the effect of decreasing the volume of the reaction mixture? Increasing the volume of the reaction mixture? Adding an inert gas at constant volume? SOLUTION The chemical equation has 3 mol of gas on the right and zero moles of gas on the left. Decreasing the volume of the reaction mixture increases the pressure and causes the reaction to shift to the left (toward the side with fewer moles of gas particles). Increasing the volume of the reaction mixture decreases the pressure and causes the reaction to shift to the right (toward the side with more moles of gas particles). Adding an inert gas has no effect. For Practice 14.15 Consider the following reaction at chemical equilibrium: What is the effect of decreasing the volume of the reaction mixture? Increasing the volume of the reaction mixture? Example 14.16 The Effect of a Temperature Change on Equilibrium The following reaction is endothermic. What is the effect of increasing the temperature of the reaction mixture? Decreasing the temperature? SOLUTION Since the reaction is endothermic, we can think of heat as a reactant: Raising the temperature is equivalent to adding a reactant, causing the reaction to shift to the right. Lowering the temperature is equivalent to removing a reactant, causing the reaction to shift to the left. For Practice 14.16 The following reaction is exothermic. What is the effect of increasing the temperature of the reaction mixture? Decreasing the temperature? Arrhenius Definition: The Simplest • What is an acid? – Acids ionize in water to produce H+ ions and anions. HCl(aq) → H+(aq) + Cl−(aq) CH3CO2H(aq) → H+(aq) + CH3CO2−(aq) • What is a base? – Bases dissociate in water to produce OH− ions and cations. NaOH(aq) → Na+(aq) + OH−(aq) • Bronsted–Lowry Definition The most general theory for common aqueous acids and bases • What is an acid? – ACIDS DONATE H+ IONS. – Similar definition as presented in Arrhenius theory • What is a base? – BASES ACCEPT H+ IONS. – This broadens the Arrhenius definition for a base. – Answers the questions about nonhydroxide bases that the Arrhenius theory can not explain. Bronsted–Lowry: Conjugate Acid/Base Pair • NH3 is a BASE in water and water is an ACID. • NH3 / NH4+ is a conjugate pair. • Related by the gain or loss of H+ • Every acid has a conjugate base. • Every base has a conjugate acid. Example 15.1 Identifying Brønsted–Lowry Acids and Bases and Their Conjugates Identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base in each reaction. SOLUTION (a) Since H2SO4 donates a proton to H2O in this reaction, it is the acid (proton donor). After H2SO4 donates the proton, it becomes HSO4−, the conjugate base. Since H2O accepts a proton, it is the base (proton acceptor). After H2O accepts the proton it becomes H3O+, the conjugate acid. (b) Since H2O donates a proton to HCO3− in this reaction, it is the acid (proton donor). After H2O donates the proton, it becomes OH–, the conjugate base. Since HCO3– accepts a proton, it is the base (proton acceptor). After HCO3– accepts the proton it becomes H2CO3, the conjugate acid. Acid Strength: Strong vs. Weak Strong Weak • COMPLETE (~100%) dissociation in water – Complete ionization – Strong electrolyte • PARTIAL (< 5%) dissociation in water – Weak electrolyte • Acid HNO3(aq) + H2O(l) H3O+ + NO3- • Acid CH3COOH(aq) + H2O(l) H3O+ + CH3COO- • Base Ca(OH)2(aq) + 2 OH- + Ca2+ • Base NH3(aq) + H2O(l) NH4+ + OH- Ka Values for Weak Acids Weak Acid Equation Ka acetic acid HC2H3O2 H+ + C2H3O2- 1.8 x 10-5 benzoic acid C6H5CO2H H+ + C6H5CO2- 6.4 x 10-5 formic acid hydrocyanic acid HCHO2 HCN H+ + CHO2- 1.8 x 10-4 H+ + CN- 6.2 x 10-10 hydrofluoric acid HF H+ + F- 7.2 x 10-4 hypochlorous acid HOCl H+ + OCl- 3.5 x 10-8 lactic acid CH3CH(OH)CO2H H+ + CH3CH(OH)CO2- 1.4 x 10-4 phenol HOC6H5 H+ + OC6H5- 1.6 x 10-10 Problem: What is the percent ionization of a 2.5 M HNO2 solution? Equation: HNO2 + H2O NO2 + H3O+ HNO2 NO2 H3O+ I 2.5 0 0 C -x +x +x E 2.5 – x +x +x Assume x <<<< 2.5 Ka = 4.6 x 10-4 = (x2/2.5) (4.6 x 10-4)1/2 = (x2/2.5)1/2 x = 3.4 x10-2 Percent ionization = (3.4 x 10-2/2.5) x 100 = 1.4% Ka = 4.6 x 10-4 Strength of Conjugate Acid-Base Pairs Water, Strong Acid, Strong Base • Water is an extremely weak electrolyte (K = 1 x 10-14). – About 1 out of every 10 million water molecules forms ions through a process called auto ionization. 2 H2O(l) H3O+ + OH– • Equilibrium constant for water’s auto ionization is given as Kw. – Kw = [H3O+] [OH-] = 1.00 x 10-14 at 25 oC • As [H3O+] increases, the [OH–] MUST decrease so that the product stays constant. – Inversely proportional relationship between H+ and OH- ion. Summary of Mathematical Relationships • pH = - log [H+] • pOH = - log [OH-] [H+] = 10-pH [OH-] = 10-pOH • pKw = -log (Kw) • pKa = -log (Ka) • pKb = -log (Kb) Kw = 10-pKw Ka = 10-pKa Kb = 10-pKb • pKw = pKa + pKb Kw = Ka x Kb • pH + pOH = 14 [H+] [OH-] = 1 x 10-14 Example 15.2 Using Kw in Calculations Calculate [OH−] at 25 ºC for each solution and determine whether the solution is acidic, basic, or neutral. SOLUTION (a) To find [OH–] use the ion product constant. Substitute the given value for [H3O+] and solve the equation for [OH−]. Since [H3O+] > [OH−], the solution is acidic. (b) Substitute the given value for [H3O+] and solve the acid ionization equation for [OH−]. Since [H3O+] < [OH−], the solution is basic. Example 15.2 Using Kw in Calculations Continued (c) Substitute the given value for [H3O+] and solve the acid ionixation equation for [OH–]. Since [H3O+] = 1.0 10–7 and [OH–] = 1.0 10–7. the solution is neutral. For Practice 15.2 Calculate [H3O+] at 25 °C for each solution and determine whether the solution is acidic, basic, or neutral. Example 15.3 Calculating pH from [H3O+] or [OH–] Calculate the pH of each solution at 25 °C and indicate whether the solution is acidic or basic. (a) [H3O+] = 1.8 10–4 M (b) [OH–] = 1.3 10–2 M SOLUTION (a) To calculate pH, substitute the given [H3O+] into the pH equation. Since pH < 7, this solution is acidic. (b) First use Kw to find [H3O+] from [OH–]. Then substitute [H3O+] into the pH expression to find pH. Since pH > 7, this solution is basic. For Practice 15.3 Calculate the pH of each solution at 25 °C and indicate whether the solution is acidic or basic. (a) [H3O+] = 9.5 10–9 M (b) [OH–] = 7.1 10–3 M Example 15.4 Calculating [H3O+] from pH Calculate the [H3O+] concentration for a solution with a pH of 4.80. SOLUTION To find the [H3O+] from pH, start with the equation that defines pH. Substitute the given value of pH and then solve for [H3O+]. Since the given pH value is reported to two decimal places, the [H3O+] is written to two significant figures. (Remember that 10log x = x (see Appendix I). Some calculators use an inv log key to represent this function.) For Practice 15.4 Calculate the H3O+ concentration for a solution with a pH of 8.37. Example 15.5 Finding the [H3O+] of a Weak Acid Solution Find the [H3O+] of a 0.100 M HCN solution. 1. Write the balanced equation for the ionization of the acid and use it as a guide to prepare an ICE table showing the given concentration of the weak acid as its initial concentration. Leave room in the table for the changes in concentrations and for the equilibrium concentrations. (Note that the H3O+ concentration is listed as approximately zero because the autoionization of water produces a negligibly small amount of H3O+.) 2. Represent the change in the concentration of H3O+ with the variable x. Define the changes in the concentrations of the other reactants and products in terms of x, always keeping in mind the stoichiometry of the reaction. Example 15.5 Finding the [H3O+] of a Weak Acid Solution Continued 3. Sum each column to determine the equilibrium concentrations in terms of the initial concentrations and the variable x. 4. Substitute the expressions for the equilibrium concentrations (from step 3) into the expression for the acid ionization constant (Ka). In many cases, you can make the approximation that x is small (as discussed in Section 14.8). Substitute the value of the acid ionization constant (from Table 15.5) into the Ka expression and solve for x. Confirm that the x is small approximation is valid by calculating the ratio of x to the number it was subtracted from in the approximation. The ratio should be less than 0.05 (or 5%). Example 15.5 Finding the [H3O+] of a Weak Acid Solution Continued 5. Determine the H3O+ concentration from the calculated value of x and calculate the pH if necessary. 6. Check your answer by substituting the calculated equilibrium values into the acid ionization expression. The calculated value of Ka should match the given value of Ka. Note that rounding errors and the x is small approximation could cause a difference in the least significant digit when comparing values of Ka. Since the calculated value of Ka matches the given value, the answer is valid. For Practice 15.5 Find the H3O+ concentration of a 0.250 M hydrofluoric acid solution. Example 15.6 Finding the pH of a Weak Acid Solution Find the pH of a 0.200 M HNO2 solution. 1. Write the balanced equation for the ionization of the acid and use it as a guide to prepare an ICE table showing the given concentration of the weak acid as its initial concentration. Leave room in the table for the changes in concentrations and for the equilibrium concentrations. (Note that the H3O+ concentration is listed as approximately zero because the autoionization of water produces a negligibly small amount of H3O+.) 2. Represent the change in the concentration of H3O+ with the variable x. Define the changes in the concentrations of the other reactants and products in terms of x, always keeping in mind the stoichiometry of the reaction. Example 15.6 Finding the pH of a Weak Acid Solution Continued 3. Sum each column to determine the equilibrium concentrations in terms of the initial concentrations and the variable x. 4. Substitute the expressions for the equilibrium concentrations (from step 3) into the expression for the acid ionization constant (Ka). In many cases, you can make the approximation that x is small (as discussed in Section 14.8). Substitute the value of the acid ionization constant (from Table 15.5 ) into the Ka expression and solve for x. Confirm that the x is small approximation is valid by calculating the ratio of x to the number it was subtracted from in the approximation. The ratio should be less than 0.05 (or 5%). Example 15.6 Finding the pH of a Weak Acid Solution Continued 5. Determine the H3O+ concentration from the calculated value of x and calculate the pH if necessary. 6. Check your answer by substituting the calculated equilibrium values into the acid ionization expression. The calculated value of Ka should match the given value of Ka. Note that rounding errors and the x is small approximation could cause a difference in the least significant digit when comparing values of Ka. For Practice 15.6 Find the pH of a 0.0150 M acetic acid solution. Missing text: Since the calculated value of Ka matches the given value, the answer is valid.




