Homework Assignment #2-Chem 102_W14 REV
advertisement

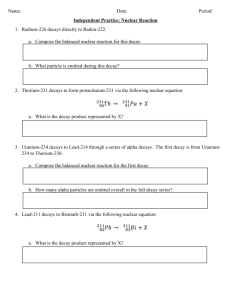
Homework Assignment #2 Chem 102 – Due 7 January 2015, by 5:00 pm 1) Based on intermolecular forces, rank the following compounds from highest to lowest melting points: butane, decane, hexane, ethane; explain why. 2) Draw the following: a) dichlorodiiodomethane b) 1,4-diethylcyclohexane c) cis-2-octene d) 1-bromo-3-chloro-1-heptyne e) 1,3,5-trifluoropentane 3) Write a balanced chemical equation for the combustion of the following: a) heptane b) cyclobutane c) octane d) 2,2,4-trimethylpentane (aka isooctane) 4) Write a balanced chemical equation for the bromination of the following (show all possible products): a) hexane b) methane c) pentane d) 2-hexene e) cis-5-octene 5) A patient receives 9.0 ng (9.0 x 10-9 g) of a radioisotope with t1/2 = 12 hrs. How much will remain in the body after 2.0 days (48 hrs.), assuming radioactive decay is the only path for removal of the isotope from the body? 6) Write a balanced nuclear equation for alpha decay of bismuth-212. 7) Complete the following nuclear equation: 190 Pt 78 +? 8) Complete the following nuclear equation: ? 21490Th + 9) Briefly describe the 3 major types of nuclear radiation. 10) What are cis/trans isomers? Which type of organic molecules can have cis/trans isomers? 11) Write the balanced chemical equation for the hydration of 1-propene. Circle the major product. 12) Write the balanced chemical equation for the hydrogenation of 3-heptene. 13) If a patient is administered 10 ng of technetium-99m, how much will remain 1 day later? Assuming radioactive decay is the only pathway whereby Tc-99m is removed from the body. 14) Draw the following: a) 1,2,4-triethylbenzene b) 1,3,4-trinitrotoluene (aka TNT; nitro group = NO2) c) 1,2-dimethylbenzene 15) What is the main use of alkanes in today’s society? 16) List the IUPAC rules for naming: alkanes, cycloalkanes and alkenes.