File
Science 10
CR3 Semi-Notes
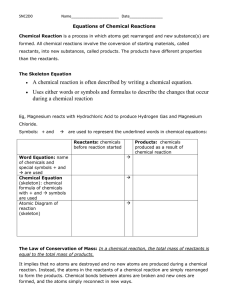
Chemical Reactions
• For every chemical reaction, we can describe it with a _____________________.
• most are _________________________ (Ag, K, Fe)
• 8 are _________________ (H
2
, N
2,
O
2,
F
2,
Cl
2,
Br
2,
I
2
, At
2
)
‘_____________________________’
• 2 are ___________________ (S
8,
P
4
)
Examples
Convert the following word equations into chemical equations.
1.
Aluminum oxide reacts with magnesium to produce magnesium oxide and aluminum.
2.
Iron (III) oxalate reacts with potassium sulfide and creates potassium oxalate and iron (III) sulfide.
3.
Hydrogen reacts with oxygen and produces dihydrogen monoxide.
4.
Dicarbon hexahydride reacts with oxygen and creates carbon dioxide and dihydrogen monoxide.
5.
Magnesium hydroxide reacts with hydrogen monochloride and creates magnesium chloride and dihydrogen monoxide.
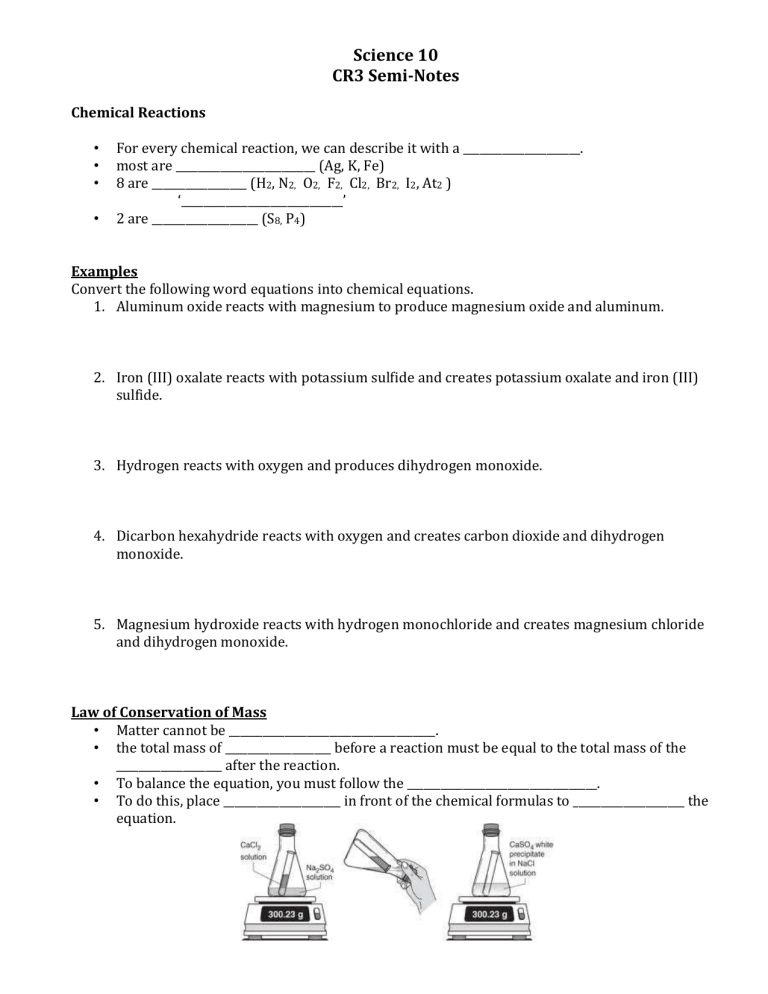
Law of Conservation of Mass
• Matter cannot be _____________________________________.
• the total mass of ___________________ before a reaction must be equal to the total mass of the
___________________ after the reaction.
• To balance the equation, you must follow the __________________________________.
• To do this, place _____________________ in front of the chemical formulas to ____________________ the equation.
Examples: a) Sodium reacts with chlorine to produce sodium chloride. b) Ammonia (NH
3
) and oxygen react to produce nitrogen dioxide and water. c) Aluminum sulfate reacts with calcium chloride to make aluminum chloride and calcium sulfate. d) Propanol (C
3
H
5
OH) burns with oxygen to produce water and carbon dioxide.
Types of Chemical Reactions a) Synthesis Reaction
The combination of ___________________________________ to form a new compound. b) Decomposition Reaction
One substance _____________________________ into 2 or more simpler ones. c) Combustion Reaction
It involves the burning of a _____________________________________. The products are usually H
2
O and CO
2
. d) Single Replacement Reaction
A free element __________________________ that is found in a compound. e) Double Replacement Reactions
Elements from ________________________________________ exchange places with one another.
f) Acid – Base Reactions (Water Forming)
In this reaction, _______________________________________________ is formed.
1.
2.
Examples: Balance the following equations and identify the reaction type.
CuCl
2
+ Ag
3
PO
4
Cu
3
(PO
4
)
2
+ AgCl
MgO Mg + O
2
4.
3. H
2
SO
4
+ LiOH H
2
O +
P
4
+ O
2
P
2
O
5
5. F
2
+
6. C
7
H
13
OH
Cr
2
O
3
CrF
3
+
+ O
2
CO
2
Li
O
+
2
2
SO
4
H
2
O