CALCULATE THE MOLAR MASS (GFM)
advertisement

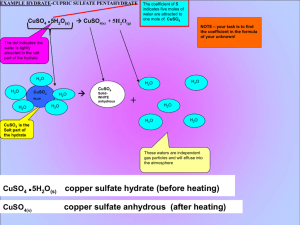
OVERVIEW OF HYDRATE CALCULATIONS 1. 1. 2. 3. 4. CALCULATION OF THE WATER OF HYDRATION COEFFICIENT FROM YOUR DATA, FIND MASS OF ANHYDROUS SALT ( SUBTRACT EMPTY BEAKER FROM BEAKER CONTAINNING THE ANHYDRATE AT END OF LAB). CALCULATE THE MOLAR MASS (GFM) OF YOUR ANHYDROUS SALT AND CONVERT YOUR MASS TO MOLES. FROM YOUR DATA, FIND MASS OF WATER LOST DURING HEATING (SUBTRACT THE MASS OF THE BEAKER CONTAINNING HYDRATE BEFORE HEATING FORM BEAKER AFTER HEATING). CALCULATE THE MOLAR MASS OF WATER AND CONVERT THE WATER TO MOLES. COMPARE THE MOLES OF ANHYDROUS SALT TO MOLES OF WATER BY DIVIDING BY THE SMALLEST, THIS SHOULD GIVE THE WATER OF HYDRATION COEFFICIENT. . SAMPLE DATA for dehydration of CuSO4 X H2O EMPTY BEAKER = 70.0 g BEAKER + HYDRATE = 72.0 g BEAKER AFTER FINAL = 71.3 g HEATING (CONTAINS ANHYDROUS SALT) 71.3g - 0.70 GRAMS WATER WAS LOST DURING HEATING. To calculate the water mass, subtract the beaker before heating (contains hydrated salt, from the beaker after heating (contains the anhydrous salt). The mass lost is water gas that escaped your beaker 70.0 g = 1.3 g OF ANHYDROUS SALT (CuSO4 ) To calculate the mass of the anhydrous (“without water”) salt simply subtract the empty beaker from the beaker after heating. After the heating cycle the hydrate has been dehydrated and is Termed the anhydrous salt. CALACULATE THE MOLAR MASS OF THE ANHYDROUS SALT CuSO4 Atomic mass element Subtotal for the element Cu 1 X 63.546 = 63.546 S 1 X 32.06 = 32.06 O 4 X 15.99 = 63.96 Formula subscripts + 159.566g/mol CALACULATE THE MOLAR MASS OF THE H2O Formula subscripts Atomic mass element H 2 X Subtotal for the element 1.00794 2.01588 = O 1 X 15.99 15.99 = + 18.00588 g/mol CALACULATE THE MOLES OF THE H 2O element Atomic mass H 2 X 1.00794 = O 1 X 15.99 = Subtotal for the element 2.01588 + 15.99 18.00588 g/mol MOLES = MASS/GFM MOLES (H2O) = 0.70g/18.00588 g/mol MOLES (H2O) = 0.0388761 mol H2O in hydrate CALACULATE THE MOLES THE ANHYDROUS SALT CuSO4 Atomic mass element Cu 1 X 63.546 Subtotal for the element 63.546 = S 1 X O 4 X 32.06 = 32.06 15.99 = 63.96 + 159.566g/mol MOLES = MASS/GFM MOLES (CuSO4) = 1.3g/156.566 g/mol MOLES (CuSO4) = 0.0083037 mol CuSO4 in hydrate MOLES (H2O) = 0.0388761 mol H2O in hydrate MOLES (CuSO4) = 0.0083037 mol CuSO4 in hydrate Now that we have the moles of the water and anhydrous salt, we divide by the smallest number of moles to get in integer ratio. 0.0388761 mol H2O = 4.68 which rounds to 5 0.0083037 mol CuSO4 Therefore the mole ratio of water to anhydrous salt is 5:1, X, the water of hydration is 5.