Chap 14 Worksheet Overall Practice
advertisement
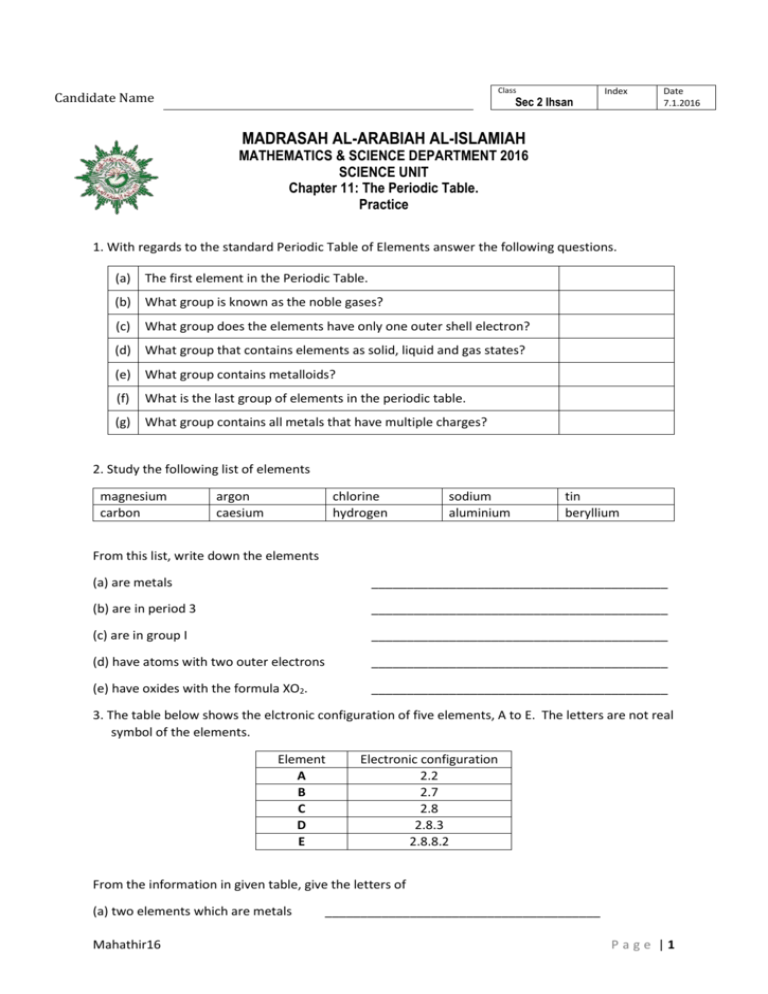
Class Candidate Name Sec 2 Ihsan Index Date 7.1.2016 MADRASAH AL-ARABIAH AL-ISLAMIAH MATHEMATICS & SCIENCE DEPARTMENT 2016 SCIENCE UNIT Chapter 11: The Periodic Table. Practice 1. With regards to the standard Periodic Table of Elements answer the following questions. (a) The first element in the Periodic Table. (b) What group is known as the noble gases? (c) What group does the elements have only one outer shell electron? (d) What group that contains elements as solid, liquid and gas states? (e) What group contains metalloids? (f) What is the last group of elements in the periodic table. (g) What group contains all metals that have multiple charges? 2. Study the following list of elements magnesium carbon argon caesium chlorine hydrogen sodium aluminium tin beryllium From this list, write down the elements (a) are metals __________________________________________ (b) are in period 3 __________________________________________ (c) are in group I __________________________________________ (d) have atoms with two outer electrons __________________________________________ (e) have oxides with the formula XO2. __________________________________________ 3. The table below shows the elctronic configuration of five elements, A to E. The letters are not real symbol of the elements. Element A B C D E Electronic configuration 2.2 2.7 2.8 2.8.3 2.8.8.2 From the information in given table, give the letters of (a) two elements which are metals Mahathir16 _______________________________________ Page |1 (b) one element which is a noble gas _______________________________________ (c) two elements in the same group _______________________________________ (d) two elements in the same period _______________________________________ 4. An element X in Period 3 of the Periodic Table has 6 outer shell electrons. (a) In which group it belongs to. Explain your answer. (b) What is the name and symbol of X? (c) How many electron shells are there in an atom of X? (d) Is the element a metal or non-metal? (e) What will be the charge and chemical symbol of its ion? 5. Strontium (Symbol Sr) is below calcium in Group II of the Periodic Table. Its is found as a ore called strontianite. The ore is similar to limestone, an ore to calcium. (a) Give the chemical name and formula of the compound in strontianite. (b) For the metal strontium, how many electrons does it have in its outermost shell? Give a reason. (c) Write down the formula of the compound strontium nitrate. Mahathir16 Page |2