BMED2801- Lecture 23: Parasympathetic Nervous System & its
advertisement

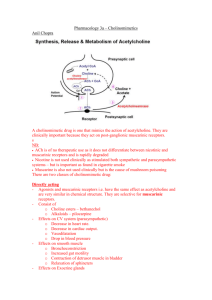
BMED2801- Lecture 23: Parasympathetic Nervous System & its’ receptors Learning objectives are to: • understand the role of the parasympathetic nervous system (PNS) in health and disease • identify the transmitters and corresponding receptors involved • understand the physiological responses associated with activation of PNS receptors in various sites of the body • describe the mechanisms of action of drugs affecting PNS function(s) Main functions of Autonomic Nervous System - The autonomic system is involved in: o Muscle contractility e.g. intestinal smooth muscle and bronchiole smooth muscle and relaxation of smooth muscle. o Controlling the heart – how fast it beats and how forcefully o Exocrine (external) secretions - for e.g. when exercising it activates the sympathetic nervous system and causes sweating o Endocrine (internal) secretions- as in when exercising you produce adrenaline form adrenal gland that is hormonal secretion and also control of insulin secretion i.e. trying to control blood sugar levels when doing activity o Various metabolic process– like breaking down fat for energy, utilization of glucose for energy in the cell - the ANS is broken down into 3 different divisions The enteric division is located in the gut and it is much more self regulatory than the other two, can function independently of the CNS Physiology of ANS • Sympathetic activity – increases in stress “fight or flight” When you are really active, stressed, exercising etc. this is when the SNS is most active Physiological changes include; Heart beat goes up, breathe more deeply, able to run faster (adrenaline), muscle contractility in the gut is slowed down, trying to mobilize energy e.g. breaking down fat for energy, using glucose stores. • Parasympathetic (ParaNS) activity predominates during repose “rest and digest” • SNS and ParaNS have some opposite actions (e.g. GI smooth muscle, heart rate) – the SNS will reduce contractility of gut and increase heart rate. Conversely, the PNS will cause peristaltic action of GI smooth muscle and decrease heart rate. • SNS and ParaNS can also have similar actions eg salivary glands – when either ParaNS or SNS are activated you can get production of saliva. • Both systems operate continuously to maintain homeostasis Note: They are both always working but to different extents depending on the condition. Transmitters involved in neuronal projections from the CNS to ganglia (collection of nerve fibres) - transmitter in both ParaNS and SNS is acetylcholine – it is binding to nicotinic receptors present on these ganglia so they are called autonomic ganglia in both divisions of the ANS Transmitters involved in neuronal projections form ganglia to the periphery (tissue) SNS - For example noradrenalin being released in SNS and that for e.g. is constricting blood vessels- and that’s why BP increases when exercising because you get release of catecholamine like adrenaline and noradrenalin which helps to raise BP. - in SNS – whilst noradrenalin and adrenaline are the main transmitters that are important in sympathetic response in the postganglionic neuron, a small percentage use Ach- that’s in the sweat glands primarily are acting on muscarinic receptors to promote the sweat being produced - In the vast majority of cases what is released is noradrenalin at the synaptic level –but at the adrenal gland – Ach acting on nicotinic receptors promote the release of noradrenalin and adrenaline from the adrenal gland which will circulate in the blood around the body to diff. tissues to produce response by interacting with adrenoceptors. ParaNS - The transmitter released is ACh acting on muscarinic receptors which is a diff. type of cholinergic receptor – producing salivation, increased GIT motility etc. Note: - the preganglionic neuron – is the neuron projecting to the ganglia the neurone projecting form the ganglia to target tissue = postganglionic neurone Summary ParaNS transmitters • Acetylcholine (Ach) acts on 2 different receptors - at the autonomic ganglia ACh is acting on nicotinic cholinergic receptors (mAChR) - at peripheral tissue the action is on muscarinic cholinergic receptors (nAChR) • Preganglionic neurons are cholinergic, ganglionic transmission occurs via nicotinic Ach receptors • Postganglionic ParaNS neurons are cholinergic, acting on muscarinic receptors in targets • There are 5 classes of muscarinic receptors The Anatomical view of the Parasympathetic Nervous System: See diagram above: This is the ParaNS Medullary outflow: - Via the medulla in the brain stem – we get outflow via cranial nerves (12 cranial nerves) – some of which are v. important to the ParaNS for e.g. - CN III – ocular motor nerve – innervates the eye - CN VII- facial nerve –innervating lacrimal and salivary gland - CN IX – glossopharyngeal nerve – innervates salivary gland (secretions when we activate this part of the ANS) - CN X – vagus neve – goes to various tissues including the heart to slow it down and also to the lung and upper gut Sacral Outflow: - This outflow comes out the bottom of the spine at the sacral level –via a nerve bundle called the nervi erigentes to the lower gut, bladder and genitals. Actions of Ach - Acetylcholine (ACh) has diverse actions on a number of cell types mediated by two major classes of receptors. • Muscarinic Receptors: - Muscarine is an agonist derived from amanita muscaria that binds v. efficiently to muscarinic receptors Muscarinic receptors are part of the transmembrane, G protein coupled receptor family. Atropine (antagonist) reverses muscarine effects Note: Transduction of the ACh message is more complex in the muscarinic family of receptors. And the family of muscarinic receptors is more complex than the nicotinic family. There are at least 5 muscarinic receptor subtypes expressed in humans. For most purposes it is sufficient to concentrate on M1, M2 and M3 receptors. M1 receptors are located in autonomic ganglia and the central nervous system. M2 receptors are located mainly in the supraventricular parts of the heart. M3 receptors are located in smooth muscles and glands, and on endothelial cells in the vasculature. • Nicotinic receptors: - nicotine derived from tobacco is also an agonist at nicotinic receptors (ligand gated ion channels) In the periphery, these are present in autonomic ganglia and neuromuscular junction (NMJ) - Tubocurarine is an antagonist (blocker) at NMJ - it competitively blocks Ach from acting at nicotinic receptors - Whereas mecamylamine is an antagonist at ganglia Note: - - Nicotine works on nicotinic receptors both in the ganglia and at neuromuscular junction With the neuromuscular junction don’t get this confused with autonomic nervous system – that’s a separates system – the neuromuscular junction is present in the somatic efferent system – which is involved in voluntary muscle contraction The autonomic nervous system is different to this. Somatic efferent system – is at the level of the skeletal muscle not the smooth muscle like in the ANS, ACh is acting on nicotinic receptors at the neuromuscular junction. There are two major subtypes of nicotinic receptors; those found in the neuromuscular junction of skeletal muscle (nicotinic muscle, Nm) and those found in autonomic ganglia and other parts of the nervous system (nicotinic neuronal, Nn). When ACh or other agonists occupy the receptor site on the external surface of the cell membrane there is a conformational change in the ion channel and an increase in conductance to the ion(s) for which that channel is selective. Thus, when Nm receptors are activated, there is an influx of cations through the ion channel and depolarization of the motor end plate. In short, nicotinic receptors rather directly transduce the ACh external messenger into an action on the cell. Muscarinic actions in PNS Muscarinic receptors– are specifically found in the paraNS, except for muscarinic receptors found on sweat glands that can be activated by sympathetic stimulation. Mediated by 5 subtypes of muscarinic receptors (MAChRs) which are G-protein coupled i.e. they have second messenger system associated with them 3 subtypes are well-characterised: • M1 “neural” • M2 “cardiac” present on the heart • M3 “glandular” tend to present on glands MAChR s- mechanisms of action M1, M3 and M5: • They act via inositol phosphate pathway (increase inositol triphosphate (IP3) and then Ca2+ release from intracellular store) this can be associated with alterations in muscular contraction. M2, M4: • Their second messenger is cyclic AMP • They act by inhibiting adenylate cyclase (decrease cAMP) within the cell Main sites & effects of MAChR – cardiovascular - - The main receptor in the paraNS present at the heart is M2 – muscarinic 2 what it does: it slows down the heart and decreases its force of contraction and also decreases the conduction velocity. If there is too much decrease in the conduction velocity we get atrioventricular block - where there is an absence of conduction between the atrium and the ventricle which could happen if there is excessive cholinergic stimulation. There are M2 receptors are also present on the nodal tissue i.e. the sinoatrial and atrioventricular nodes as well as atrial muscle, but there is no significant effect at the ventricular muscle. Blood vessels in the cardiovascular system - The main place M3 are present are in the penis and can cause erectile dilatation. Main sites & effects of MAChR -bronchi, GI - - At the lungs on the bronchiole smooth muscle there are M3 receptors – they mediate contraction of the bronchiole smooth muscle causing potential bronchoconstriction and glandular secretion GI – activate M3 receptors present here – you get increased motility i.e.; peristaltic movements which moves food along and helps with digestion, sphincter dilatation allowing food to pass from one part of the intestine to another and also impt. in maintain normal bowel motility There are also M3 receptors on glands within the gut which are responsible for glandular secretion In the stomach there are also M1 receptors – which are associated with gastric acid secretion when activated. Main sites & effects of MAChR - bladder, glands, eye - - The paraNS is impt. for maintaining one’s ability to urinate – need to be able to contract detrusor muscles and relax the sphincter in the bladder so urine can pass out – this happens via Ach activating M3 receptors Lacrimal glands and salivary glands -M3 receptors – will cause secretion of tears and saliva. In the Eye – M3 receptor mediated – causing papillary constriction and ciliary muscle contraction. -Ach gets stored in vesicles - These vesicle release Ach into the synapse via exocytosis -Ach can then interact with the post synaptic cholinergic receptors in the paraNS- the cholinergic receptor type are muscarinic receptors causing secretions, slowing of the heart, gastric motility etc. -Inactivating enzyme acetylcholinesterase which will break down the ACh Synthesis and transport of Acetylcholine: - Synthesis of acetylcholine is facilitated by the enzyme, choline acetyltransferase (CAT). This enzyme combines choline with acetate derived from acetyl coenzyme A (CoA). - ACh in cholinergic nerve fibers is taken up into synaptic vesicles and released by exotyosis so transmitter can get into the synapse. this uptake process is inhibited by the drug vesamicol - - Release of acetylcholine, like synaptic release at other junctions, is based on quantal release of vesicles containing preformed neurotransmitter molecules. Vesicular release depends on depolarization of the nerve terminal and the influx of calcium ion. In ways not yet understood in detail, the influx of calcium promotes simultaneous exocytosis of many vesicles. At the motor end-plate in the neuromuscular junction this results in a relatively massive release of ACh (hundreds of vesicles and thousands of ACh molecules per vesicle) and an end-plate potential that normally results in depolarization of the muscle cell and contraction. The release of ACh at various cholinergic junctions can be blocked by certain toxins, most notably those produced by Clostridium species. Botulinum toxin A, from Clostridium botulinum binds to cholinergic nerve terminals and is internalized. Once internalized it acts on the vesicle release process and prevents exocytosis. All junctional release of ACh is inhibited by such toxins. In patients poisoned by Clostridium botulinum the immediate clinical problem is flaccid paralysis and respiratory failure. Metabolism of acetylcholine Inactivation of acetylcholine: -Acetylcholine (ACh) is terminated by hydrolysis, which is greatly accelerated by one or more of the cholinesterase –enzymes. - Acetylcholinesterase (Ache) is present in high concentration in cholinergic synapses – it is the main cholinesterase –enzyme. - Butyrylcholinesterase, also known as pseudocholinesterase is another cholinesterase –enzyme that helps in the breaking down of ACh. - Hydrolysis of ACh produces choline which is recycled and taken back into the never terminal and then made into ACh which can then be restored in the vesicles. Cholinergic drug types • Anticholinesterases* and other drugs enhancing cholinergic transmission prevent the breakdown of ACh so there can be a higher level of ACh in the synapse to act on cholinergic receptors • Muscarinic agonists increase the ACh transmission to act on cholinergic receptors • Muscarinic antagonists block cholinergic activity in the paraNS • Ganglion-stimulating drugs tend to act more preferentially at the autonomic ganglia to stimulate nicotinic receptors • Ganglion-blocking drugs tend to act more preferentially at the autonomic ganglia to block nicotinic receptors • Neuromuscular-blocking drugs* Block neuromuscular transmission at the neuromuscular junction, causing paralysis of the affected skeletal muscles *For more detail see BMED2806 lecture I gave on Poisoned Arrows and Muscle Paralysis Drugs – different mechanisms affecting cholinergic transmission Increase cholinergic transmission: - Use an enzyme inhibitor to inhibit the breakdown of ACh e.g. neostigmine Use: Treatment of myasthenia gravis – a disease that causes the production of abnormal antibodies that damage or block many of the muscle receptors for ACh. This prevents ACh from binding to the muscle receptors - Mimic the action of Ach at the receptor using agonist such as muscarine or nicotine - Enhance Ach release using drugs such as 4-aminopyridine Decreasing cholinergic transmission: - Inhibit the synthesis of ACh using a blocker of synthesis called hemicholinium. Impair the storage of ACh in synaptic vesicles - Impair the release of Ach e.g. botox and high doses of magnesium - disrupts the vesicular release of ACh. - Block Ach from binding to receptor – use drugs such atropine to block muscarinic receptors or tubocurarine to block nicotinic receptors. Drugs enhancing cholinergic transmission • Can either inhibit cholinesterase (mainly) or increase ACh release Types of Cholinesterases: • Acetylcholinesterase • Butyrylcholinesterase = pseudocholinesterase • Anticholinesterases: There are different types of cholinersterase inhibiting drugs (anticholinesterase) which will enhance cholinergic transmission via increase of ACh levels in the synapse: -Short acting cholinesterase inhibitor (edrophonium) used to test for my myasthenia gravis (muscle weakness), they give the patient edrophonium and test muscular activity ½ later – if there is improvement in muscle strength – then probable diagnosis = myasthenia gravis. - Intermediate acting anticholinesterase (neostigmine) 2-4hrs half life – must be taken a few times a day - Irreversible cholinesterase inhibitor (parathion) used for controlling pests, poisoning people as a toxin. Effects of anticholinesterases = cholinesterase inhibitors Inhibiting cholinesterase activity Enhances action of ACh at receptors • ANS: - Bradycardia (slowing of the heart) – because we are boosting Ach at M2 receptors - hypotension (low BP) - Hypersecretion – because there is more ACh stimulating M3 receptors associated with glandular secretion - Bronchoconstriction because there is an increase in ACh stimulating M3 receptors in bronchiole smooth muscle causing a constrictor response - GIT hypermotility mediated by muscarinic receptor activation because there is too much ACh in the synapse – causing cramping and pain - Decease intraocular pressure – via muscarinic receptors in the eye. • NMJ: - Not only will you get too much stimulation of muscarinic receptors but you also get too much stimulation of nicotinic receptors at the neuromuscular junction = fasciculations, inc. twitch tension, depolarisation block (e.g. suxamethonium depolarizing blocker) • CNS: - convulsions, unconsciousness, respiratory failure with organophosphates (long acting anticholinerase inhibitors) i.e. parathion • Antidote: early pralidoxime within 2hrs – otherwise the effects are irreversible. Some clinical uses of anticholinesterases In anesthesia the neostigmine can be used to reverse paralysis from neuromuscular blockade actionblock acetylcholiesterase enzyme, thereby increase ACh levels, and thereby restoring function via activation of nicotinic receptors at the neuromuscular junction. - In myasthenia gravis – autoimmune mediated loss of activity in nicotinic receptors in neuromuscular junction – use endrophonium to test for the disease and then oral neostigmine to treat the disease – end result: reduction in muscular weakness Get muscarinic side effects –because it is not selective for the neuromuscular junction, ACh level is being increased everywhere – therefore it will activate muscarinic receptors = side effects however, habituation occurs and effect is lessened. - Muscarinic agonists - - Carbachol is an agonist that acts on cholinergic receptors specifically muscarinic receptors – only difference between carbochol and ACh – is the amino group added instead of methyl group on ACh – the structure is fairly similar that’s why it can act as an agonist at those receptors – more specifically because of that slight change that makes it more selective. Methylcholine –in comparison to ACh there is an extra methyl group. Bethanechol= has a structure like carbochol but has got an extra methyl group added to it Slight structural changes – are enough to make these drugs work more on muscarinic receptors – hence, they are more selective Whereas ACh works on both muscarinic and nicotinic Also, muscarine it has retained a portion of the ACh structure – the end structure is different- hence making it more selective to muscarinic receptors • Ach, muscarine, carbachol Clinical application: - e.g. Pilocarpine (muscarinic agonist)- for glaucoma (to decrease intraocular pressure) the drug acts on muscarinic receptors to cause aqueous humor to drain properly and decrease pressure - Topical use of the drug e.g. eye drops - you can still get some absorption into the blood stream through the capillaries = muscarinic receptor systemic effects on- GI, glands Muscarinic antagonists (blockers) • They are competitive – they compete with ACh to bind to the receptor • Similar structure to ACh but acetyl group is replaced by an aromatic group – this ring structure allows it to act as a blocker instead of an agonist. • Naturally occurring in alkaloids (solanaceous plants) and lipid soluble tertiary ammonium compounds • Atropine (from deadly nightshade Atropa belladonna) – blocks muscarinic receptors • Hyoscine (from thorn apple plant Datura stramonium) Effects of atropine (opposite to ACh!) - - It will block secretion, slow heart down, dilate pupils, increase pressure in your eyes, relax the smooth muscle of the bladder causing retention of urine instead of secretion, reduce motility of GIT causing cramping and actions on the bronchiole, biliary and urinary smooth muscle In the CNS – make you restless, agitated, confused, disoriented and hyperactive. Clinical uses of antimuscarinics (muscarinic antagonist or blocker) - Atropine- In the heart, use of atropine IV – after heart attack heart beats way too slowly giving atropine will block ACh – blocks slowing of heart, which means the heart rate will increase - Tropicamide a short acting agent to dilate pupils during ophthalmic examination of the eye - Hyoscine used to treat motion sickness - Benztropine muscarinic blocker specifically in the brain used in Parkinson’s disease and also treatment of antiphychotic agent mediated movement disorders - Ipotropiane/tiatropiame modern derivative – Used for asthma – used to relax bronchiole smooth musclewhen you activate muscarinic receptors of bronchiole on smooth muscle it causes constriction this drugs is doing the opposite, dilation. - Anaesthesia premed: eg. atropine (to dry respiratory tract secretions) long operations can cause a lot of build up of secretion in respiratory tract which can lead to infections such as pneumonia. Atropine will inhibit muscarinic receptors thereby inhibiting secretion. - GI uses: -For “antispasmodic”effects i.e. in irritable bowel syndrome (too much contractility of the gut), eg. Hyoscine butylbromide (Buscopan) to relax smooth muscle – inhibit the contractility of the gut. -Suppress gastric acid secretion e.g. pirenzepine- selective M1 antagonist located in the stomach responsible for gastric acid secretion – they act to decreases these secretions - used for peptic ulcer disease (less used now). Ganglion stimulants - tobacco – nicotine • Nicotinic agonists can affect receptors in both autonomic ganglia (periphery) and motor end-plate receptors (at neuromuscular junction, NMJ) • But nicotine from tobacco plant and DMPP are relatively more selective for autonomic ganglionic receptors Ganglion-blocking drugs Several mechanisms can: • Interfere with ACh release (hemicholinium, BOTOX) so get ganglion block (no further transmission form autonomic ganglia to the periphery) & skeletal muscle paralysis • Produce prolonged depolarisation. I.e. Nicotine initially stimulates then blocks. – Too much stimulation for a long period = blockade. • Interfere with postsynaptic action of ACh. Do this by blocking nicotinic receptors/assoc. ion channels • Main effects: hypotension (decrease in BP), loss in cardiovascular reflexes, inhibit secretion (because there is no transmission beyond ganglia to the periphery to control glandular secretions), GI paralysis and constipation, impaired urination. Neuromuscular blocking agents: • Clinical use: adjunct to anaesthesia to induce muscle paralysis • These do not act in parasympathetic system; instead they work in somatic efferent system involving the neuromuscular junction. • Most are non-depolarising blocking agents (competitive antagonists of ACh at nicotinic receptors). • Examples of NM blocking agents: Curare alkaloids in poison arrows (tubocurarine), SE hypotension • Synthetic agents: i.e. pancuronium long acting • Actions: low BP, paralysis in surgery, blurred vision, hard to swallow NM blocking agents: Depolarising blocking drugs • Agonists at ACh receptor i.e. suxamethonium – agonist at the cholinergic receptor. • Acts to cause prolonged depolarisation of motor endplate leading to loss of electrical excitability hence, causing blockade this is functional antagonism • Broken down by acetyl cholinesterase • SE: bradycardia (slowing of the heart), increased intraocular pressure, prolonged paralysis