The GATE approach Sept 2015 Oxford
advertisement
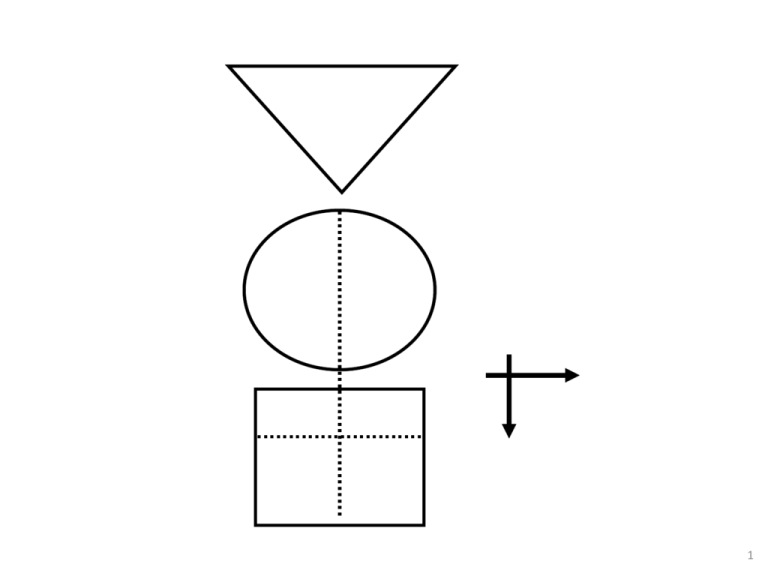
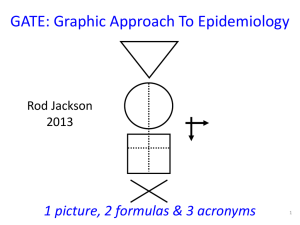
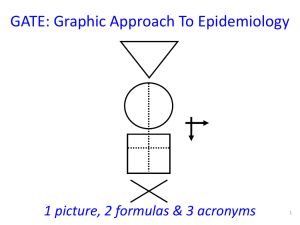
1 GATE: Graphic Appraisal Tool for Epidemiology 1991 - 2015 1 picture, 2 formulas & 3 acronyms 2 GATE: Graphic Appraisal Tool for Epidemiology Graphic Architectural Tool for Epidemiology Graphic Approach To Epidemiology making epidemiology accessible 3 4th year medical students 1991 Jerry Morris numerator epidemiology = denominator In: Uses of Epidemiology 1957 5 presentation outline GATE is a framework for: 1. study design 2. study analysis 3. study error 4. practicing EBM 1 picture, 2 formulas & 3 acronyms 7 GATE: a framework for study design 1 picture every epidemiological study can be hung on the GATE frame 1 picture, 2 formulas & 3 acronyms 9 1 picture: GATE frame cohort of British doctors smoking status allocated by measurement (observation) smokers yes lung cancer events counted no non-smokers followed for 10 years cohort / longitudinal / follow-up study 1 picture, 2 formulas & 3 acronyms 10 1st acronym: PECOT British doctors P Participants randomly allocated to aspirin or placebo Exposure E C Comparison aspirin placebo Outcomes yes MI no O T Time 5 years randomised controlled trial 1 picture, 2 formulas & 3 acronyms 11 middle-aged Americans P body mass index measured overweight diabetes status measured in all participants yes no E C ‘normal’ weight O T cross-sectional (prevalence) study 12 middle-aged American women P receive mammogram screening test mammogram positive breast cancer E C mammogram negative yes O no T diagnostic test (prediction) study 13 middle-aged American women P Gold Standard breast cancer positive mammogram negative test E C O no breast cancer T diagnostic (test accuracy) study 14 P smokers lung cancer E C non-smokers smoking status measured cases yes O no controls T case-control study (all nested in virtual cohort studies) 15 £100 GATE: a framework for study analysis: 1st formula: occurrence = outcomes ÷ population the numbers in epidemiological studies can be hung on the GATE frame 1 picture, 2 formulas & 3 acronyms 18 1st formula: occurrence of outcomes = number of outcomes ÷ number in population/group British doctors P Participant Population smoking status measured Exposure Group EG CG Comparison Group smokers Outcomes Lung cancer non-smokers yes no a O b T Time 10 years 19 Population P British doctors smoking status measured Exposure Group EG CG Comparison Group smokers Outcomes Lung cancer non-smokers yes no a O b T Time 10 years Exposure Group Occurrence (EGO) = a÷EG = number of outcomes (a) ÷ number in exposed population (EG) 20 P British doctors Population randomly allocated Exposure Group EG CG Comparison Group aspirin Outcomes MI placebo yes no a O b T Time 5 years Comparison Group Occurrence (CGO) = b÷CG = number of outcomes (b) ÷ number in comparison population (CG) 21 Epidemiology = Numerator ÷ Denominator middle-aged American women P Participant Population receive mammogram screening test D Exposure Group EG mammogram positive Outcomes yes breast cancer no a Comparison Group mammogram negative N T Time O 22 the goal of all epidemiological studies is to calculate EGO and CGO P British doctors smoking status measured smokers yes EGO: Occurrence (risk) of no cancer in smokers EG a CG non-smokers O b Lung cancer 10 years T CGO: Occurrence of cancer in nonsmokers 23 P Middle-aged Americans Body Mass Index (BMI) measured High BMI EG CG Low BMI high low EGO: Average blood glucose in EG O CGO: Average blood glucose in CG 24 P Middle-aged Americans Body Mass Index (BMI) measured High BMI blood glucose high low E C Low BMI O T cross-sectional study with numerical measures 25 P Middle-aged American women Gold Standard Breast cancer positive mammogram negative EGO: likelihood of a positive mammogram if breast cancer E C O no Breast cancer T CGO: likelihood of a positive mammogram if no breast cancer 26 1st formula: occurrence = outcomes ÷ population its all about EGO and CGO • EGO ÷ CGO = Relative Risk (RR) • EGO – CGO = Risk Difference (RD) measures of occurrence: risk; rate; likelihood; probability; average; incidence; prevalence 27 GATE: framework for nonrandom error 2nd acronym: RAMBOMAN Recruitment Allocation Maintenance Blind Objective Measurements ANalyses 1 picture, 2 formulas & 3 acronyms 29 Study setting RAMBOMAN Eligible population recruitment process P P Recruitment of participants ‘who are the findings applicable to?’ 30 RAMBOMAN: ‘were participants well Allocated to exposure & comparison groups?’ was Allocation to EG & CG successful? RCT: allocated by randomisation (e.g to drugs) Cohort: allocated by measurement (e.g. smoking) EG & CG EG CG similar at baseline? EG CG O T E&C measures accurate? O T 31 P RAMBOMAN ‘were Participants well Maintained in the groups they were allocated to?’ EG CG O T completeness of follow-up compliance contamination co-interventions 32 P EG CG RAMBOMAN ‘were outcomes well Measured?’ were they measured Blind to whether participant was in EG or CG ? O T 33 RAMBOMAN P ‘were outcomes well Measured?’ were they measured Objectively? EG CG O T 34 RAMBOMAN P EGA ‘were the ANalyses done well?’ CGA EGC CGC If RCT were Intention To Treat (ITT) analyses done? b a O T 35 P RAMBOMAN ‘were the ANalyses done well?’ EG CG adjustment for baseline differences / confounding? O T 36 GATE: random error: 2nd formula: random error = 95% confidence interval sample from a population EGO ± 95% CI CGO ± 95% CI There is about a 95% chance that the true value in the underlying population lies within the 95% CI (assuming no non-random error) 1 picture, 2 formulas & 3 acronyms 37 GATE: a framework for error in systematic reviews & meta-analyses: 3rd acronym: FAITH 1 picture, 2 formulas & 3 acronyms 38 systematic review: a study of studies study sources studies screened studies appraised & allocated: included excluded studies summarised & pooled if homogeneous 39 critical appraisal of SR: FAITH study sources Find studies screened Appraise studies appraised & allocated: Include included Total Heterogeneity? excluded studies summarised & pooled if homogeneous 40 1 picture, 2 formulas & 3 acronyms GATE: framework for the 4 steps of EBP 42 the steps of EBP: 1. Ask 2. Acquire 3. Appraise 4. Apply & Act 43 EBP Step 1: ASK - turn your question into a focused 5-part PECOT question P 2. Exposure 4. Outcomes E yes no 1. Participants C 3. Comparison O T 5. Time 44 EBP Step 2: ACQUIRE the evidence – use PECOT to help choose search terms P Exposure Outcomes E yes no Participants C Comparison O T Time 45 EBP Step 3: APPRAISE the evidence – with the picture, acronyms & formulas P Recruitment P Allocation E Maintenance E C blind objective O T C O T Measurements ANalyses Occurrence = outcomes ÷ population Random error = 95% Confidence Interval 46 APPLY the evidence by AMALGAMATING the relevant information & making an evidencebased decision:’ the X-factor © 47 48 X-factor: making evidence-based decisions epidemiological evidence person values & preferences family community system features economic legal political patient’s clinical circumstances practitioner X Practitioner e pertise: ‘putting it all together’ - the art of practice Clinical expertise in the era of evidence-based medicine and patient choice. EBM 2002;736-8 (March/April) 49 GATE critically appraised topic (CATs) forms find these at: www.epiq.co.nz GATE CAT – 4-sheet workbook (in Excel) sheet 1: GATE-Ask & Acquire 51 GATE CAT – 3-sheet workbook (in Excel) sheet 2: GATE-Appraise (with calculator) 52 GATE CAT – 3-sheet workbook (in Excel) sheet 3: GATE-Apply 53 56