Topic: Indicators
advertisement
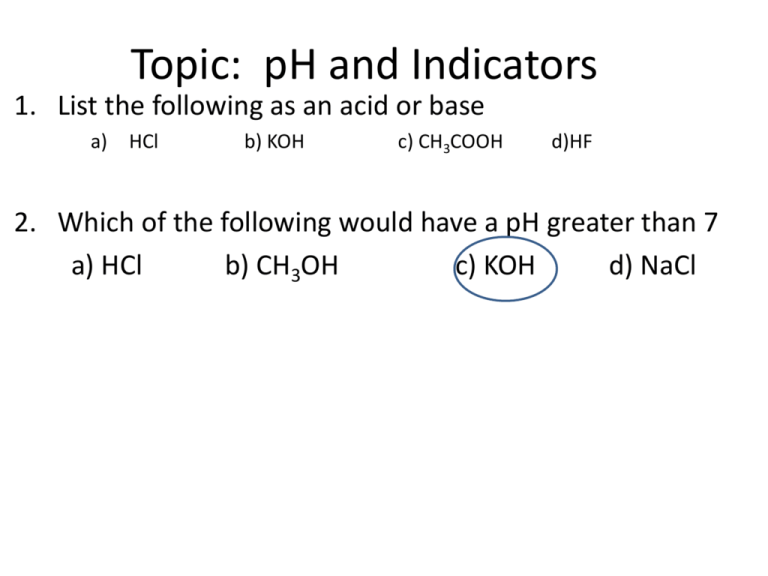
Topic: pH and Indicators 1. List the following as an acid or base a) HCl b) KOH c) CH3COOH d)HF 2. Which of the following would have a pH greater than 7 a) HCl b) CH3OH c) KOH d) NaCl pH scale power (or potential) of Hydrogen Ranges from 0-14 measures H+ concentration [H+] the more H+, the more acidic the solution Acid, Base, or Neutral • Neutral solution: pH = 7 [H+] = [OH-] • Acidic solution: pH LESS THEN 7 H+1 > OH-1 • Basic solution: pH GREATER THEN 7 OH-1 > H+1 How to safely test pH • NEVER “taste” • Instruments – use a pH meter • See if the substance reacts with a metal other than Cu, Ag, or Au • Indicators – use a series of indicators Indicator • substance that changes color over narrow pH range What color would the following indicators be in a neutral solution? yellow green colorless purple blue yellow Use several indicators to narrow down pH range of substance Ex: Three samples of the same solution are tested, each with a different indicator. All three indicators, bromthymol blue, bromcresol green and thymol blue, appear blue The pH of the solution must be greater than_____ Practice using table M 1. What indicator is yellow with a pH 9.8 2. Which indictor is blue with a pH of 5.6 0-3.0Orange3.1.-4.4 Green Pink Purple 0-3.7 3.8-5.4 Green Green 4.5-14 7.7-14 8.4-14 5.5-14 9.7-14