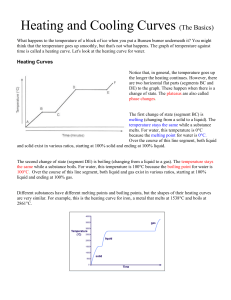
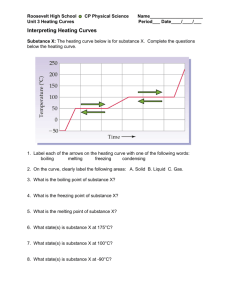
Heating and Cooling Curves
advertisement

Heating and Cooling Curves Paris Thatos Partner: Alan Delaney Partner: Mikey Christopher Due 10/26/09 PreLab: The transfer of heat is indicated by measuring its effect on the temperature of the surroundings (one example being the water in a calorimeter). No direct measurements of the substance undergoing the heat transfer are made. IN this experiment, we will observe, by direct measurement, the effects of heating and cooling a pure substance. Purpose: To study the effects of heating and cooling a pure substance through change of phase. To also construct heating and cooling curves based on observational data. And to determine the freezing and melting point of the substance. Materials: Refer to lab pages 89 – 90 for materials and equipment. One change in the equipment was used: lauric acid was substituted out for steric acid. Safety: Do not come into contact with or inhale vapors of the steric acid. Avoid consumption Tie back long hair and loose clothing when using the Bunsen burner. Heat safety goggles in the laboratory. If using a mercury thermometer, take caution. The mercury is highly poisonous and can be deadly if the thermometer breaks. Procedure: Obtain a test tube of steric acid and use a beaker of water to construct a hot water bath. Once the steric acid has fully melted, put a thermometer into the acid and begin keeping track of the time. Record the temperature every 30 seconds until the emperature drops to 45 degrees Celsius. If there is adequate time, remove from source of heat and begin taking measurements every 30 seconds until the temperature reaches 80 degrees Celsius Observations: Time (minute) Temp (degrees Celsius) 0 83 0.5 78 1 74 1.5 68 2 65 2.5 64 3 62 3.5 60 4 59 4.5 57 5 56 5.5 54 6 53 6.5 53 7 53 7.5 53 8 53 8.5 53 9 53 9.5 53 10 53 10.5 53 11 53 11.5 53 12 53 12.5 53 13 53 13.5 53 14 53 14.5 53 15 53 15.5 53 16 53 16.5 53 17 53 17.5 53 18 53 18.5 52 19 52 19.5 52 20 51 20.5 51 21 51 21.5 51 22 51 22.5 50 23 50 23.5 50 24 49 24.5 49 25 49 25.5 48 26 47 26.5 47 27 46 27.5 45 Calculations: Construct a cooling curve with the data: Inadequate time to run experiment needed to construct heating curve Questions: 1. Referring to your graph, determine the freezing point of steric acid. How does this temperature compare with the melting point of the same substance as indicated by your graph? The freezing point of steric acid is 53 degrees Celsius. There was inadequate time to complete the heating curve, but the melting point would be the same temperature. 2. Explain the diagonal parts of the cooling curve in terms of changes in kinetic and potential energy The diagonal parts of the graph are the times where kinetic energy and the potential energy of the substance change. 3. What phase changes are exothermic? Endothermic? The endothermic phase changes are melting and boiling. The exothermic phase changes are freezing and condensing. 4. In which phase of a substance do the particles have the greatest amount of kinetic energy. The phase of a substance where the molecules have the greatest amount of kinetic energy is the gas phase. Conclusions: Heating and cooling curves can be used to accurately tell us important information about certain substances. We can use them to tell the melting points and boiling points of many pure substances. That, and the fact that they can be easily constructed; make heating and cooling curves an important aspect of chemistry.