exam 3 worksheet - Iowa State University
advertisement

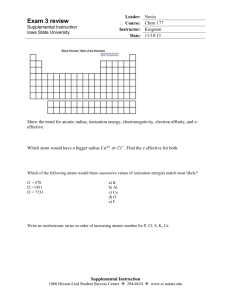
Leader: Course: Instructor: Date: Exam 3 review Supplemental Instruction Iowa State University Nevin Chem 177 Kingston 11/18/13 Show the trend for atomic radius, ionization energy, electronegativity, electron affinity, and zeffective. Which atom would have a bigger radius 𝐶𝑎2+ 𝑜𝑟 𝐶𝑙 − Which of the following atoms would these successive values of ionization energies match most likely? I1 = 578 I2 =1451 I3 = 7733 a) K b) Al c) Ca d) O e) F Write an isoelectronic series in order of increasing atomic number for P, Cl, S, K, Ca Supplemental Instruction 1060 Hixson-Lied Student Success Center 294-6624 www.si.iastate.edu Which of the two shows the electron affinity energy change a) 𝑁𝑎(𝑔) → 𝑁𝑎+ + 𝑒 − b) 𝑁𝑎(𝑔) + 𝑒 − → 𝑁𝑎− Complete and balance the reactions 𝐾2 𝑂 + 𝐻2 𝑂 → 𝐶𝑂2 + 𝑁𝑎𝑂𝐻 → 𝑁𝑖𝑂 + 𝐻𝑁𝑂3 → What electronegativity difference would a bond be considered non polar, polar, and ionic? Which has higher lattice energy? NaCl or CaCl_2 What is the relationship between lattice energy and Atomic radius? Draw the lewis dot structure for the following molecules: Find Formal charges, show net polarity. 𝑁2 𝑆𝑂42− Finish the statement. Elements past group ______ are capable of accommodating more than 8 electrons. Estimate the bond enthalpy for the following reaction: 2 𝐶𝐻4 (𝑔) + 𝑂2 (𝑔) → 2𝐶𝐻3 𝑂𝐻(𝑔) 1) 2) 3) 4) 5) 6) CH4 Draw the lewis structures Show net polarity Show hybridization of each bond List how many Sigma and Pi bonds there are Write the geometry Predict the bond angles SF6 𝐶𝑙2 𝐶𝑂 NO3 – Organic Compound (Only label the hybridization of the bonds) Draw an MO Diagram for OF +, find the bond order, label as paramagnetic or diamagnetic. What assumptions must be made to use Charles’ Law, Boyle’s Law, and Gay-Lussac’s Law? What is the molar mass of a gas in a balloon that can hold 5 L with 20g of gas with 800 mm Hg of pressure and 38 degrees C?