Chapter 11 Notes
advertisement
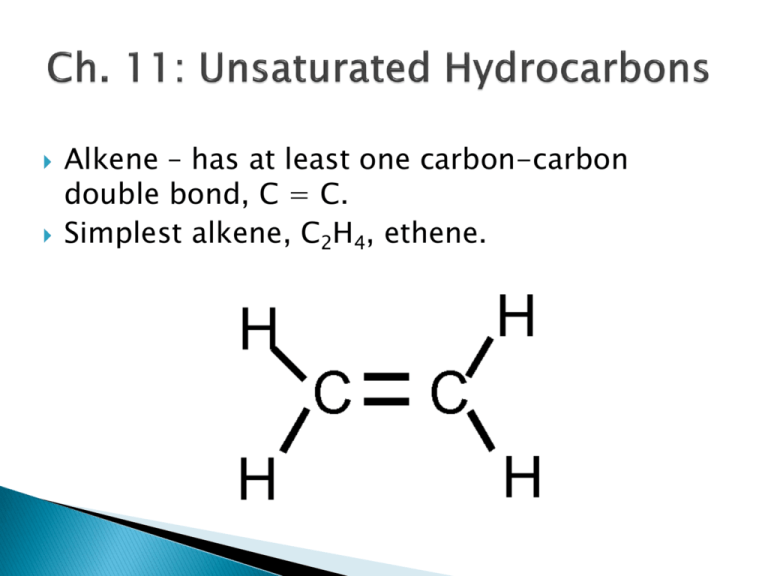
Alkene – has at least one carbon-carbon double bond, C = C. Simplest alkene, C2H4, ethene. Alkyne – has at least one carbon-carbon triple bond, C C. Simplest alkyne, C2H2, ethyne. Can also have double bond with ring structure. Cycloalkene – a ring with one or more carbon-carbon double bond. Naming an alkene. 1. Find the longest chain that includes the double bond. Base name uses –ene suffix. 2. Number the chain so that the double bond gets the lowest numbers (has priority over other substituents). Only four carbons or longer will need a number for the double bond position. Use only the lowest number for start of double bond. 3. Number substituents based on this numbering. 4. Cycloalkenes – the double bond is ALWAYS position #1 and #2. 5 4 3 2 1 CH3 – CH – CH2 – CH = CH2 CH3 Correct: 4-methyl-1-pentene Not correct: 2-methyl-4-pentene Correct = 3-methylcyclopentene Not Correct = 1-methylcyclopentene LEP #1 The double bond in an alkene consists of a sigma and a pi bond. Pi bonds do NOT allow free-rotation. C2H6 (Ethane) versus C2H4 (Ethene). The molecule around that double bond is planar (flat). The molecule 2-butene has two unique structures. CH3 – CH = CH – CH3 How? LEP #2 The molecule 1-butene does not! CH2 = CH – CH2 – CH3 Many alkenes do not have a pair of geometric isomers. Requires two different sets of groups on each side of the double bond – one large and one small. A B C=C B A Opposite = trans, Same side = cis LEP #3 These types of molecules are found in many places and are very important. Ex) Bombykol – a sex attractant (pheremone) for the silkworm moth. Both alkenes and alkynes can undergo an addition reaction where the double bond is broken and two atoms or molecule fragments are added to each side of the double bond. Hydrogenation, adding H2 with a catalyst. Halogenation, adding Cl2, Br2, or I2. Hydration, adding H2O (H-OH) with an acid catalyst. LEP #4 A polymer is a long chain of repeating units called monomers. Analogy: train = monomers are the individual cars. Monomers are typically small alkenes. Initiated by organic peroxide, R-O-O-R’, which is split into two fragments, 2 R-O Benzoyl peroxide Each fragment has an odd electron. Peroxide fragment then reacts with monomer unit using one electron from double bond. Monomer end now has odd electron, which is used to react with a second monomer. Process repeats thousands of times. CH2=CH2, ethylene makes polyethylene. ◦ Two forms – low density and high density ◦ Uses: CH2=CHCl, vinyl chloride makes PVC. ◦ Uses: CH2=CHCH3, propene makes polypropylene. ◦ Uses: CF2=CF2, tetrafluoroethene makes Teflon. ◦ Uses: CH2=CCl2, 1,1-dichloroethene makes Saran. ◦ Uses: CH2=CH(C6H5), phenylethene makes polystyrene. ◦ Uses: Recycling – uses a series of symbols and numbers to identify the type. C6H6 = Benzene A ring structure, but very different from the cycloalkanes. Three double bonds alternate with every other C-C in ring. Alkenes are reactive because of the double bond. Benzene, though, is not reactive at all. Why? True structure is an average of the two below. The six electrons from the three double bonds are delocalized (free to move). Chemists often show this by drawing the hexagon and putting a circle inside to represent these electrons. Some common mono-substituted benzene molecules have common names that are allowed by IUPAC rules. OH CH3 Benzene as a substituent (C6H5-) is called Phenyl. Mono-substituted benzenes do not require a number. Di-substituted benzenes do require numbers. A phenol or toluene will always have the –OH or – CH3 group as position #1. LEP #5 Br





