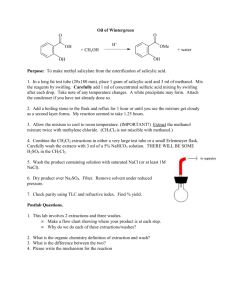
3-Nitrobenzaldehyde-1, 1-diacetate (Entry 10) 2
advertisement

S1 Functionalized poly(amidoamine) dendrimer as a strong ionic Brønsted acid organocatalyst for protection/deprotection of aldehydes Ali Pourjavadi*, Seyed Hassan Hosseini Polymer Research Laboratory, Department of Chemistry, Sharif University of Technology, Tehran, Iran E-mail: purjavad@sharif.edu Supplemental Materials EXPERIMENTAL Synthesis of acidic functionalized PAMAM (PAMAM@SO3H) Typically, a solution of ethylenediamine (2.5 g, 0.041 mol) in methanol (10 mL) was added drop wise to a solution of methyl acrylate (17.5 g, 0.203 mol) in methanol (20 mL) at 0 C and mixture was stirred for 1 h. Then mixture was stirred for further 3 d at room temperature. The volatile materials were removed under reduced pressure at 40 C. The resulting product was dissolved in methanol (20 mL) and was added drop wise to a solution of ethylenediamine (37.56 g, 0.625 mol) in methanol (50 mL) at 0 C. The resulting solution was allowed to warm to room temperature and stirred for a further 7 d at room temperature. Methanol was evaporated using a rotary evaporator at 40 C and excess ethylenediamine was removed by an azeotropic mixture of toluene and methanol with volume ratio 9/1. The remaining toluene was removed by azeotropic distillation using methanol. Methanol was evaporated under vacuum to provide the aminoterminated product as yellow oil (G0). The next generation of PAMAM (G1) was prepared following the same manner, using a 50-fold excess of methyl acrylate and a 125-fold excess of ethylenediamine with respect to the amount of amino groups. S2 PAMAM (G1) (1.0g, 0.70 mmol) was dissolved in dry dichloromethane (30 mL) and ClSO3H (1 mL, 15mmol) was drop wise added to this solution at 0 C over a period of 15 min. The mixture was stirred for further 1h at room temperature until evolution of HCl gas was completely stopped. The resulting precipitate was filtered and washed with CH2Cl2 to remove excess ClSO3H. Afterward, yellow precipitate was dissolved in 5 mL water and mixture was stirred for 2h. Then, mixture was drop wise added into the 50 mL of diethyl ether and the resulting solid products were filtered as PAMAM@SO3H. Protection and deprotection of aldehydes catalyzed by PAMAM@SO3H To a magnetically stirred solution of aldehyde (1 mmol) and freshly distilled acetic anhydride (5 mmol), PAMAM@SO3H (10 mg) was added at room temperature, and the mixture was stirred until complete disappearance of aldehydes (as monitored by TLC). After completion of reaction, CH2Cl2 was added and the catalyst was extracted and dried at 50°C for another run. The organic layer was washed with saturated NaHCO3 (3 × 25 mL) and water (15 mL), and dried over anhydrous Na2SO4. Evaporation of the solvent under reduced pressure and recrystallization from ethanol gave the almost pure 1,1-diacetate. For deprotection of corresponding 1,1 diacetates 0.5 mmol of prepared acylal and PAMAM@SO3H (5 mg) was stirred in methanol (2mL) at room temperature. The solvent was evaporated and catalyst was extracted by CH2Cl2. The organic layer was washed with saturated NaHCO3 (3 × 25 mL) and water (15 mL), and dried over anhydrous Na2SO4. Evaporation of the solvent under reduced pressure and recrystallization from ethanol gave the almost pure aldehyde. 1 H NMR details of selected compounds in Table 3 are shown below. S3 4-Chlorobenzaldehyde-1, 1-diacetate (Entry 2)1: 1H-NMR (CDCl3, δ ppm): 2.16 (s, 6H), 7.41 (d, 2H), 7.49 (d, 2H), 7.66 (s, 1H). 4-Nitrobenzaldehyde-1, 1-diacetate (Entry 9) 1: 1H-NMR (CDCl3, δ ppm): 2.18 (s, 6H), 7.74 (d, 2H), 7.76 (s, 1H), 8.3 (d, 2H). 3-Nitrobenzaldehyde-1, 1-diacetate (Entry 10)2: 1H-NMR (CDCl3, δ ppm): 2.19 (s, 6H), 7.63 (t, 1H), 7.76 (s, 1H), 7.87 (d, 1H), 8.3 (d, 1H), 8.43 (d, 1H). 4-Acetoxy-3-methoxybenzaldehyde-1, 1-diacetate (Entry 14) 2: 1H-NMR (CDCl3, δ ppm): 2.12 (s, 6H), 2.30 (s, 3H) 7.13 (d, 2H), 7.54 (d, 2H), 7.67 (s, 1H). 4-(1,1-diacetoxymethane)benzaldehyde-1,1-diacetate (Entry 16)1: 1H-NMR (CDCl3, δ ppm): δ 2.15 (s, 6H), 7.59 (s, 2H), 7.70 (s, 1H). S4 Figure S 1.:FT-IR spectrum of (a) PAMAM and (b) PAMAM-sulfamic acid functionalized Figure S 2: The TGA curves of PAMAM (G1) and PAMAM@SO3H S5 Figure S3. 1H NMR of catalyst S6 Table S 2: Elemental analysis of catalyst Catalyst C% H% N% S% Theoretical 28.0 5.3 13.7 16.9 Experimental 28.6 5.5 14.1 16.3 1. Jin, T. S.; Sun, G.; Li, Y. W.; Li, T. S. Green Chem. 2002, 4, 255-258 2. Karimi, B.; Maleki, J. J. Org. Chem. 2003, 68, 4951-4954.