5' primer - Davidson College
advertisement

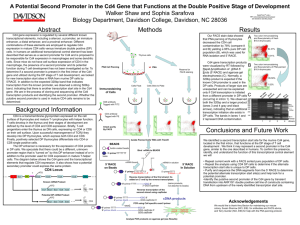
A Potential Second Promoter in the Cd4 Gene that Functions at the DP Stage of T-cell Development Walker Shaw and Sophia Sarafova Biology Department, Davidson College, Davidson, NC 28036 QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Background Methods CD4 is a protein expressed on certain cells in the immune system, predominantly T- lymphocytes. These T-cells develop by entering the thymus as a Double Negative T-Cell Progenitor (no CD4 or CD8 expressed on their cell surface). In the thymus they develop into Double Positive T- Cells (both CD4 and CD8 expressed on cell surface), before differentiating into Single Positive CD4 (T-helper) or CD8 (T-cytotoxic) cells in the immune system. N Results Our FACS stain data showed that PNA panning of thymocytes decreased the CD4 cell contamination by 78% (compare A and B) yielding a 95% pure DP cell population (B), which was used for RNA isolation and 5’ RACE. Thymus cells PNA Cell Panning PNA Reserve for FACS staining DN CELL N Cells Float DP CELL Cells Stick to Plate Immunostaining of Cells A Two color immunostaining and flowcytometry of fresh B10.A thymocytes 84.37% 6.91% Cd4 gene transcription products were visualized by RT followed by Rapid Amplification of cDNA 5’ Ends (5’ RACE) and agarose gel electrophoresis (C). Normally, a 320bp product is expected if the known Cd4 promoter is used in the DP cells. Products of larger size are unexpected and can be explained only if Cd4 transcription is initiated from a different promoter in DP cells (occuring in intron 1). We observed 1000 900 800 both the 320bp and a larger product 700 600 500 (lanes 3 and 4 gray and black 400 arrows), indicating that an additional 300 transcription initiation site exists in 200 DP cells. The bands in lanes 1 and 100 2 represent DNA contamination. B Two color immunostaining and flowcytometry of PNA-panned B10.A thymocytes 95.58% 1.52% C CD8 antibody Tagged with FITC N N CD8 CELL CD4 CELL CD4 antibody Tagged with PE Throughout T-cell development, CD4 expression is regulated by several different known transcriptional elements, including a silencer, a promoter, a DP enhancer, a distal enchancer, and a proximal enhancer. In DP cells, it has been demonstrated that the DP enhancer is necessary for the expression of CD4 on the cell membrane. We speculate that there may be a different, unknown promoter region that is “turned on” by this DP enhancer and other transcriptional elements that are different from the promoter used for CD4 expression in mature T-helper cells. … P Ex1 Sil TATA Ex2 Ex3 Ex4 Ex5 DPe Isolate Panned Cells’ RNA with Trizol® BEADS Avidin-coated beads Bind mRNA to BEADS … Known mRNA Product ATG Ex1 Ex2 Ex3 Ex4 Ex5 Potential DP mRNA Product ATG Ex2 Ex3 Ex4 Ex5 … … 5’ RACE on Beads mRNA bound 5’ primer TTTTTTT-3’ 3’-AAAAAAA-----5’ CD4 We would like to thank Amy Becton for maintaining our mouse colony, Susan Sharrow (NCI, EIB) for antibodies and FACS advice, and Terry Guinter (NCI, EIB) for help with the PNA panning protocol. mRNA in solution 5’-primerTTTT 3’AAAA------5’ (same products, not attached to beads) 5’-primer TTTT------------CCC-3’ 3’-GGG-capfinder CD4 5’ RACE In Solution Reverse transcription of the first strand; Cs added on 3’ end by the reverse transcriptase. Reverse transcription of the second strand using capfinder 5’-primer TTTT------------------CCC-3’ 3’-primer AAAA -----------------GGG-capfinder-5’ Acknowledgements 1 2 3 4 Primer dimers Importance of Findings 5’-primer TTTTTTT biotin ATG 320bp Check purity By FACS CD4 Locus ? 500bp Future Work • Investigate strategies for further purification of DP cells cDNA products PCR using Cd4 specific exon3 primer exon3 5’-primer TTTT--------------------------------CCC-3’ 3’-primer AAAA -------------------------------GGG-capfinder-5’ GGG-capfinder The CD4 gene in humans has a transcriptional control region in intron 1 that is speculated to function as a second promoter. This second promoter is thought to be responsible for CD4 expression in human macrophages. In mice, macrophages do not express CD4 however, implying that a second promoter does not exist. Our findings indicate that there is initiation of transcription starting 3’ of the known promoter in DP cells, presumably in intron 1. We think that this evidence supports the theory that there is a second promoter in the murine Cd4 gene that functions to support CD4 expression specifically in DP cells. We speculate that the human and murine second promoters may be related. exon3----capfinder exon3----capfinder Analyze PCR products on agarose gel (see Results) • Repeat the analysis using CD4 SP cells to determine if the alternate transcription start site is unique to DP cells • Purify and sequence the DNA segments from the 5’ RACE to determine the potential alternate transcription start site(s) and help look for a potential promoter