Solid Liquid
advertisement

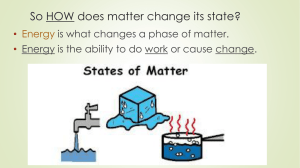
So HOW does matter change its state? • Energy is what changes a phase of matter. © 2013 S. Coates What is energy? Energy is the ability to do work or cause change. Kinetic Energy Kinetic Energy is the energy of motion Particles with a lot of kinetic energy Particles with a lot of kinetic energy move fast and far apart (a gas) Particles with little kinetic energy move slow & close together (a solid) Particles with little kinetic energy Temperature Temperature is the average kinetic energy of the individual particles in a substance So… if it is hot, it has more kinetic energy, if it is cold, it has less kinetic energy. Changing states Matter can change from one state to another when thermal energy is released or absorbed. This is called a change of state. Melting The change from the solid state to the liquid state is melting. The temperature at which a substance changes from a solid to a liquid is called the melting point. Melting is when matter absorbs energy, and its temperature rises. Freezing The change from the liquid state to the solid state is called freezing. The temperature at which a substance changes from the liquid state to the solid state is called the freezing point. Energy is released (decreases) during freezing. After all of the liquid has become a solid, the temperature begins to decrease again. Vaporization The change from a liquid state to a gas state is known as vaporization. The temperature of the substance does not change during vaporization. However, the substance absorbs thermal energy. Vaporization Two forms of vaporization exist. Vaporization that takes place below the surface of a liquid is called boiling. The temperature at which a liquid boils is called the boiling point. Vaporization that takes place at the surface of a liquid is called evaporation. Evaporation • Evaporation, which occurs at temperatures below the boiling point, explains how puddles dry up. • It takes more than speed for water molecules to escape the liquid state. • During evaporation, these faster molecules also must be near the surface, heading in the right direction, and they must avoid hitting other water molecules as they leave. Condensation As a gas cools, its particles slow down. When particles move slowly enough for their attractions to bring them together, droplets of liquid form. This process, which is the opposite of vaporization, is called condensation. The opposite of vaporization is condensation, which occurs when a gas loses enough thermal energy to become a liquid. Where does the water on the outside of the glass come from? Sublimation • Some substances can change from the solid state to the gas state without ever becoming a liquid. During this process, known as sublimation, the surface particles of the solid absorbs enough energy to become a gas. Picture from http://www.ehow.com/how_2098268_fogsmoke-dryice-halloween.html Deposition Deposition moving directly from a gas to a solid state The opposite of sublimation State Change Pyramid Absorbing thermal energy Gas Releasing thermal energy Melting Solid Freezing Liquid Fill in the Blank Phase change HW 1. liquid Complete the Chart 2. solid # Change of State 3. freezes 1 Solid to Liquid 4. 0 C, 32 F 2 Liquid to Gas 6. water vapor 3 Gas to Liquid 7. evaporates 4 Liquid to Solid 8. dry ice 5 Solid to Gas 6 Gas to Solid 5. gas 9. liquid 10. 100 C, 212 F Rise in Temp Drop in Temp Phases of Matter • ADDED Is ENERGY being ADDED or TAKEN AWAY in this phase change: The added energy has caused the chocolate particles to speed up. Before they were vibrating in place, now they are moving fast enough to slip past one another. Solid © 2013 S. Coates Liquid Phases of Matter • Is ENERGY being ADDED or TAKEN AWAY in this phase change: ADDED The added energy has caused the water particles to speed up. Before they were moving fast enough to slip past one another, now they have enough energy to break away from one another and expand. Liquid © 2013 S. Coates Gas Phases of Matter • Is ENERGY being ADDED or TAKEN AWAY in this phase change: Taken Away Taking away energy from a puddle slows the water molecules down so that they no longer slide past one another. © 2013 S. Coates Liquid Solid So What? How can I use this information? Iceland sits on the boundary of two platesthe North American Plate and the Eurasian Plate. Is it a divergent, convergent, or transform boundary? What will we see happening at this type of boundary? The 1973 Heimaey eruption started on 23 January with the opening of a 1600 m long fissure on the east side of the island, about 400 m east of the outskirts of the town. During the first hours lava issued from the entire fissure in 50-150 m high glowing fountains. A day later the eruption continued from two craters, and on 6 February only one crater remained, where activity continued until 26 June, when the eruption came to an end. By that time 417 houses had been destroyed by lava and tephra and the remainder of the town was covered with millions of tons of tephra. The total volume of the eruption products was 250 million cubic metres (230 million m3 as lava and 20 million m3 as tephra). The chemical composition of the products is alkali basalt, murgearite to hawaiite. The 1973 eruption on the island of Heimaey is a classic example of the struggle between man and volcanoes. With a heroic effort the people of Iceland saved the town of Vestmannaeyjar and the country's most important fishing port. Except where noted, all photographs are by the late Svienn Eirikksen, fire marshal of the town of Vestmannaeyjar. Photographs courtesy of the U.S. Geological Survey. The island of Heimaey with the growth of the island in 1973, the location of the eruptive fissure, and the location of Eldfell, the 1973 cone. Modified from Williams and Moore, 1983. Not only did the tremendous efforts save the port they actually improved it. The residents returned to rebuild their town and even used the heat from the cooling lava to construct a district heating system. This vertical aerial photograph of the island shows the improved harbor, Helgafell and Eldfell cones, and the new land added to the island. Photograph by Iceland Geodetic Survey, September, 8, 1973. From Williams and Moore, 1983. http://www.youtube.com/watch?v=ghl33n26d44 The Cooling of Lava State Change Pyramid Absorbing thermal energy Gas Releasing thermal energy Melting Solid Freezing Liquid Phase Change Lab 1. Get goggles and bins from the back. 2. Once your group is ready, ice and dry ice will be placed in your petri dish. 3. Make observations about the materials and answer the questions on the back of your paper. 4. Fill the beaker ½ way with water. 5. Using your gloves, drop the dry ice into the water. 6. Observe the gas being produced- which way is the gas flowing? Conclusion Questions 1. & 2. What observations did you make about dry ice and water? 3. How are the two substances similar? 4. How are they different? 5. What phase change did you observe for each? Dry Ice _________________ Frozen Water _______________ 6. Why is it called “dry ice”? 7. What happens to the ice molecules as they change to a liquid? The water molecules (2 hydrogen and one oxygen) stay together as a molecule, the elements do NOT separate from one another! 8. What happens to the dry ice molecules as they change to a gas? ~Molecules spread apart and move faster. 9. Are Ice and liquid water the same thing? Explain 10. How could you change the liquid water back to ice? Metal Ring Demo- How will the molecules of the metals change as they are heated? Slime Time! Is slime a solid or a liquid? Background Information: The three states of matter we are studying are ______. _______, and ________ Molecules in the __________ stage move very little and have a definite shape. Molecules in the ____________ phase move around and slide past each other and take the shape of their container Molecules in the __________ phase are bouncing around freely and do not have a constant volume or definite shape.