Cellular Respiration

Cellular Respiration
Cellular Respiration: The Big Picture
We are energy beings – cellular respiration is the process by which we gain energy.
We normally run aerobic cellular respiration in which we harvest energy from organic compounds using oxygen (O
2
).
The molecule of choice for fuel is glucose.
C
6
H
12
O
6
+ 6O
2
6CO
2
+ 6H
2
O
Glucose has an abundance of energy in its bonds.
We must release it in a series of small REDOX reactions so the energy that is released does not increase cell temperature too much or the proteins may freak out!
Cellular Respiration: The Big Picture
There are a variety of ways to carry out cellular respiration and not all of them require oxygen to assist in the breakdown of glucose.
– Obligate Aerobes – They require oxygen to oxidize organic molecules to make energy.
– Obligate Anaerobes – They oxidize inorganic molecules without oxygen to gain energy. (O
2 kills!)
– Facultative Anaerobes – They oxidize inorganic molecules with or without oxygen.
Note the type of molecule being oxidized – organics give big energy boosts while inorganics do not yield much energy.
Cellular Respiration: The
Details
AKA: The Hard Part!
Cellular Respiration: The Details
Aerobic cellular respiration is as follows…
C
6
H
12
O
6
+ 6O
2
6CO
2
+ 6H
From this, we can gather that…
2
O
– cellular respiration involves breaking the bonds in glucose to make six carbon dioxide molecules.
– the hydrogens get torn off of glucose to make water.
– the free energy released in the breakdown of glucose is harnessed to make ATP.
So how do we do these jobs?…
The Reactions We’ll See
1.
2.
3.
4.
5.
There are several types of reactions that we will encounter along the way.
REDOX – Electrons being taken from one and added to another.
Phosphorylation/Dephosphorylation – The adding/removal of a phosphate group (PO
4
).
Carboxylation/Decarboxylation – The adding/removal of a carbon.
Hydration/Dehydration - The adding/removal of a water molecule (H
2
O).
Isomerization – Making a molecule into its isomer – same parts, different arrangement.
Order of Operations
Here is what we have to do…in order…
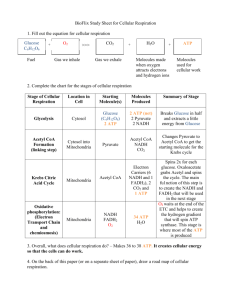
1. Glycolysis – Cytosol – Glucose splitting.
2. Pyruvate Oxidation – Matrix – Connect to
Kreb’s a la pyruvate Acetyl-CoA
3. Kreb’s Cycle – Matrix – Energy given off mainly as NADH and FADH
2
.
4. Electron Transport Chain (ETC) –
Mitochondrial inner membrane – NADH &
FADH2 give their energy to the ETC to create a proton problem.
5. Chemiosmosis – Mitochondrial inner membrane – We solve the proton problem and get a big bunch of ATP while we’re at it.
1. Glycolysis
Glycolysis means “sugar splitting”. It occurs in the cytoplasm.
Glucose goes through a series reactions that see it eventually turning into two pyruvate molecules.
These will go on to the next stage.
We get a net gain of 2 ATP and 2 NADH. The ATP are ready to use and the NADH goes in our back pocket for later.
Overall…
Glucose 2 Pyruvate
(2 ATP & 2 NADH)
2. Pyruvate Oxidation
The pyruvate goes into the mitochondrion and makes it way to the matrix.
Once in the matrix, the pyruvate is oxidized and turned into Acetyl-CoA (which will start the next stage).
Along the way, a NAD becomes an NADH which we will put in our back pocket for later.
Glucose gave us 2 pyruvate so we will end up with 2 Acetyl-CoA and 2 NADH’s.
Overall…
2 Pyruvate 2 Acetyl-CoA (2 NADH)
Pyruvate Oxidation
3. Kreb’s Cycle
The two Acetyl-CoA molecules made by the previous stage now enters a cyclical series of reactions called the Kreb’s cycle.
For each glucose, the Kreb’s cycle turns twice and we will get…
– 2 ATP
– 6 NADH
– 2 FADH
2
The ATP are used immediately and the
NADH and FADH
2 molecules will go in our back pocket for later.
The Kreb’s Cycle
4. The Electron Transport Chain
The ETC is basically a conga line of REDOX reactions
– pass the electrons to the right everybody! It all takes place on the mitochondrial inner membrane.
Electrons from NADH and FADH pumps along the way.
2 are passed into the chain and are handed down the line – hitting proton
The proton pumps take protons (H + ) from the matrix and pump them across the membrane into the intermembrane space of the mitochondrion.
This is a problem as an electrostatic & pH gradient is set up which is no good for the mitochondrion.
NADH operates 3 proton pumps while FADH operates just 2 proton pumps.
2
5. Chemiosmosis
Most texts put this step and the ETC together as one for good reason.
The proton problem created by the ETC is relieved by an enzyme, found embedded in the mitochondrial inner membrane, called ATP
Synthase.
ATP Synthase allows the protons (H + ) to come back into the mitochondrion – we’re saved!
But wait!…It gets better…ATP synthase fixes the problem and in doing so, it makes ATP!
Its like a carpenter saying, “Yeah, I can fix your roof but you have to let me pay you for it!”.
The ETC & ATP Synthase
The ATP Balance Sheet
Here’s how we get the 36 ATP from one molecule of glucose.
– 1 ATP = 1 ATP (ATP is ATP already!)
– 1 NADH = 3 ATP (goes through 3 H + pumps)
– 1 FADH2 = 2 ATP (goes through 2 H + pumps)
This gives us 38 ATP! What the…?!?!?
The trick is the 2 NADH’s made in glycolysis – in the cytosol. They have to get into the mitochondrion first and when they enter it, they are converted into
FADH
2
’s.
So what is the bottom line?
Stage of
Cellular
Respiration
The Bottom Line!
Molecule
Produced
Glycolysis 2 ATP
2 NADH 2 FADH
2
Pyruvate
Oxidation
2 NADH
Kreb’s Cycle
2 ATP
6 NADH
2 FADH
2
# of ATP
Produced
2 ATP
4 ATP
6 ATP
2 ATP
18 ATP
4 ATP
THE GRAND TOTAL 36 ATP