atomic theory
advertisement
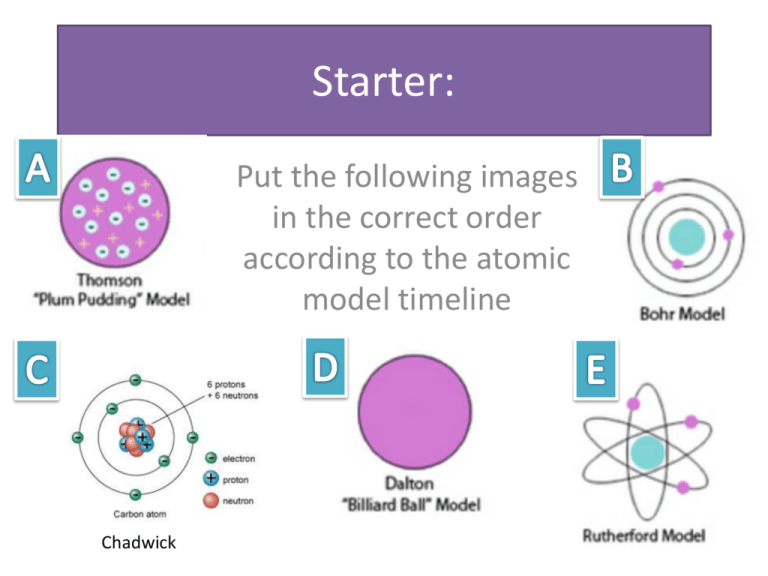
Starter: Put the following images in the correct order according to the atomic model timeline Chadwick Schedule 1. House Keeping- Missing assignments and presentations 2. Review Activity (Development of the atomic Theory) 3. Short Notes 4. Bohr Rutherford Model of the Atom 5. Atom Project Research and Plan 6. ** Build if there is time and we have the materials By the end of the lesson… I can identify the key point of Dalton’s Atomic Theory I can draw a Bohr- Rutherford model of the atom Fact Sheet • Caleb and Jack Presentation • Lea • Joshua • Joey and Jacob Advertisements: Need to be emailed! I only have Joey & Jacob L, Lea and Mo & Jamie Atomic Theory The main principles of atomic theory: 1) All matter is made up of tiny particles called atoms 2) Atoms are made up of even smaller particles called protons, neutrons, and electrons 3) Atoms can be distinguished from each other by their number of protons, neutrons, and electrons 4) A set of atoms, each of which has the same number of protons, is called an “element” 5) Atoms combine to form molecules Bohr Rutherford Diagrams A way of representing atoms “shells” represent areas in which electrons are likely to be found Each shell can contain a certain number of electrons 1st shell- 2 electrons The rest- 8 electrons Electrons are added in a particular order to the diagram Atom Building Project Build a 3-D model of an atom that includes Number of protons, electrons, and neutrons Nucleus Electron Shells Correct number of electrons in each shell If you are in partners build 2! BE CREATIVE!!! Ideas!! For the Rest of the Period… 1. Use your Ipads to research ideas for your project 2. Discuss who will bring what 3. Pick your element 4. Draw your Bohr-Rutherford Diagram 5. Begin building if possible





