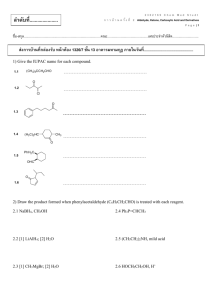
Carboxylic Acids - Bonham Chemistry
advertisement

Carboxylic Acids Chapter 18 Carboxylic Acids • In this chapter, we study carboxylic acids, another class of organic compounds containing the carbonyl group. • The functional group of a carboxylic acid is a carboxyl group, which can be represented in any one of three ways. O C-OH COOH CO2 H Nomenclature IUPAC names • For an acyclic carboxylic acid, take the longest carbon chain that contains the carboxyl group as the parent alkane. • Drop the final -e from the name of the parent alkane and replace it by -oic acid. • Number the chain beginning with the carbon of the carboxyl group. • Because the carboxyl carbon is understood to be carbon 1, there is no need to give it a number. Nomenclature • In these examples, the common name is given in O parentheses. 1O 6 3 OH Hexanoic acid (Caproic acid ) 1 OH 3-Methylbu tanoic acid (Isovaleric acid) • An -OH substituent is indicated by the prefix hydroxy-; an -NH2 substituent by the prefix amino-. OH 5 O 1 OH 5-Hydroxyhexan oic acid H2 N COOH 4-A min ob enzoic acid Nomenclature • To name a dicarboxylic acid, add the suffix -dioic acid to the name of the parent alkane that contains both carboxyl groups; thus, -ane becomes -anedioic acid. • The numbers of the carboxyl carbons are not indicated because they can be only at the ends of the chain. O HO 2 O 1 3 OH HO O 1 OH O Ethan edioic acid Prop aned ioic acid (Malonic acid ) (Oxalic acid ) O HO 4 O 5 1 OH O Butaned ioic acid (Succinic acid) HO O O 1 OH Pen tanedioic acid (Glutaric acid) HO 6 1 OH O Hexan edioic acid (Ad ipic acid) Nomenclature IU PAC N ame Structure HCOOH CH3 COOH CH3 CH2 COOH CH3 (CH2 ) 2 COOH CH3 (CH2 ) 3 COOH CH3 (CH2 ) 4 COOH CH3 (CH2 ) 6 COOH CH3 (CH2 ) 8 COOH CH3 (CH2 ) 1 0 COOH CH3 (CH2 ) 1 2 COOH CH3 (CH2 ) 1 4 COOH CH3 (CH2 ) 1 6 COOH CH3 (CH2 ) 1 8 COOH Common N ame (acid) methanoic formic ethan oic acetic propanoic propionic bu tanoic bu tyric pen tanoic valeric hexan oic cap roic octanoic cap rylic decanoic cap ric dodecanoic lauric tetradecan oic myristic hexad ecanoic palmitic octadecanoic stearic eicosan oic arachid ic D erivation Latin : formica, ant Latin : acet um, vinegar Greek: propion, firs t fat Latin : buty rum, b utter Latin : valere, to be s trong Latin : caper, goat Latin : caper, goat Latin : caper, goat Latin : laurus , laurel Greek: my ris tikos, fragrant Latin : palma, palm tree Greek: st ear, solid fat Greek: arachis, p eanut Nomenclature For common names, use, the Greek letters alpha (a), beta (b), gamma (g), and so forth to locate substituents. O C-C-C-C-OH g b a 4 3 2 1 O O H2 N 4 g 2 1 OH OH a OH 4-A min ob utanoic acid 2-Hyd roxypropan oic acid (g-A min obu tyric acid; GABA) (a-Hydroxyprop ion ic acid; lactic acid) Physical Properties hydrogen bondin g betw een tw o molecules H3 C O + H O C C O H + O - CH 3 Physical Properties Carboxylic acids are more soluble in water than are alcohols, ethers, aldehydes, and ketones of comparable molecular weight. Boilin g Solubility Molecular Poin t Weigh t (°C) (g/100 mL H 2O) Structu re N ame CH3 COOH CH3 CH2 CH2 OH CH3 CH2 CHO acetic acid 60.5 1-prop anol prop anal CH3 (CH2 ) 2 COOH butan oic acid CH3 (CH2 ) 3 CH2 OH 1-pentan ol pentan al CH3 (CH2 ) 3 CHO 60.1 58.1 118 97 48 infinite infinite 16 88.1 88.1 86.1 163 137 103 infinite 2.3 slight HA + H2O Ka larger Ka = A– + H3O+ [A–] [H3O+] [HA] increased [H3O+] stronger acid HA + H2O Ka larger Ka = A– + H3O+ [A–] [H3O+] [HA] increased [H3O+] stronger acid HA + H2O Ka larger Ka = A– + H3O+ [A–] [H3O+] [HA] increased [H3O+] stronger acid HA + H2O Ka larger Ka = A– + H3O+ [A–] [H3O+] [HA] increased [H3O+] stronger acid RCOOH + Ka H2O = RCOO– [RCOO–] [H3O+] [RCOOH] + H3O+ RCOOH + Ka H2O = RCOO– [RCOO–] [H3O+] [RCOOH] + H3O+ Comparative acidities of 0.1 M aqueous solutions of representative acids HA HCl HOAc PhOH EtSH EtOH HOH [H3O+], M Ka % ionized ~1 107 ~100 1.8 10–5 3.3 10–10 1.3 0.0036 1.3 10–3 3.6 10–6 2.88 5.44 2.5 10–11 0.0016 1.6 10–6 5.80 1.3 10–16 0.0001 1.0 10–7 7.00 1.8 10–16 0.0001 1.0 10–7 7.00 ~0.1 acids > phenols ~ thiols > water ~ alcohols pH 1.00 Fatty Acids Table 18.3 The Most Abundant Fatty Acids in Animal Fats, Vegetable Oils, and Biological Membranes. Carbon Atoms: Double Bonds * Structure Saturated Fatty Acids 12:0 CH3 ( CH2 ) 1 0 COOH Common Name Melting Point (°C) lauric acid 44 14:0 CH3 ( CH2 ) 1 2 COOH myris tic acid 58 16:0 CH3 ( CH2 ) 1 4 COOH palmitic acid 63 18:0 CH3 ( CH2 ) 1 6 COOH s tearic acid 70 arachidic acid 77 CH3 ( CH2 ) 1 8 COOH 20:0 Unsaturated Fatty Acids 16:1 CH3 ( CH2 ) 5 CH= CH( CH2 ) 7 COOH 18:1 CH3 ( CH2 ) 7 CH= CH( CH2 ) 7 COOH palmitoleic acid 18:2 oleic acid CH3 ( CH2 ) 4 ( CH= CHCH2 ) 2 ( CH 2 ) 6 COOH linoleic acid 18:3 CH3 CH2 ( CH= CHCH2 ) 3 ( CH 2 ) 6 COOH 20:4 CH3 ( CH2 ) 4 ( CH= CHCH2 ) 4 ( CH 2 ) 2 COOH arachidonic acid linolenic acid * The firs t number is the number of carbons in the fatty acid; the s econd is the number of carbon-carbon double bonds in its hydrocarbon chain. 1 16 -5 -11 -49 Fatty Acids Unsaturated fatty acids generally have lower melting points than their saturated counterparts. COOH S tearic acid (18:0) (mp 70°C) COOH Olei c acid (18;1) (mp 16°C) COOH Linoleic acid (18:2) (mp-5°C) COOH Linolenic acid (18:3) (mp -11°C) Fatty Acids Saturated fatty acids are solids at room temperature. • The regular nature of their hydrocarbon chains allows them to pack together in such a way as to maximize interactions (by London dispersion forces) between their chains. COOH COOH COOH COOH COOH Fatty Acids In contrast, all unsaturated fatty acids are liquids at room temperature because the cis double bonds interrupt the regular packing of their hydrocarbon chains. COOH COOH COOH COOH COOH Soaps • Natural soaps are sodium or potassium salts of fatty acids. • They are prepared from a blend of tallow and palm oils (triglycerides). • Triglycerides are triesters of glycerol. • The solid fats are melted with steam and the water insoluble triglyceride layer that forms on the top is removed. 21 Soaps Preparation of soaps begins by boiling the triglycerides with NaOH. The reaction that takes place is called saponification (Latin: saponem, “soap”). Boiling with KOH gives a potassium soap. 22 Soaps Figure 18.2 In water, soap molecules spontaneously cluster into micelles, a spherical arrangement of molecules such that their hydrophobic parts are shielded from the aqueous environment, and their hydrophilic parts are in contact with the aqueous environment. 23 Soaps Figure 18.3 When soaps and dirt, such as grease, oil, and fat stains are mixed in water, the nonpolar hydrocarbon inner parts of the soap micelles “dissolve” the nonpolar substances. 24 Soaps • Natural soaps form water-insoluble salts in hard water. • Hard water contains Ca2+, Mg2+, and Fe3+ ions. 25 Detergents The problem of formation of precipitates in hard water was overcome by using a molecule containing a sulfonate (-SO3- ) group in the place of a carboxylate (-CO2-) group. • Calcium, magnesium and iron salts of sulfonic acids, RSO3H, are more soluble in water than are their salts of fatty acids. • Following is the preparation of the synthetic detergent, SDS, a linear alkylbenzenesulfonate (LAS), an anionic detergent. 26 Detergents • Among the most common additives to detergents are foam stabilizers, bleaches, and optical brighteners. 27 Decarboxylation • Decarboxylation: The loss of CO2 from a carboxyl group. • Almost all carboxylic acids, when heated to a very high temperature, will undergo thermal decarboxylation. O • decarboxylation + CO2 to moderate RHresistant RCOH Most carboxylic acids, however, are high temperature heat and melt and even boil without undergoing decarboxylation. • An exception is any carboxylic acid that has a carbonyl group on the carbon b to the COOH group. Decarboxylation • Decarboxylation of a b-ketoacid. O O O b a OH 3-Oxobutanoic acid (Acetoacetic acid) warm + CO 2 Aceton e • The mechanism of thermal decarboxylation involves (1) redistribution of electrons in a cyclic transition state followed by (2) keto-enol tautomerism. enol of a keton e O H O (1) O (A cyclic six-membered transition state) O H O C O (2) O + CO 2 Decarboxylation • An important example of decarboxylation of a b-ketoacid in biochemistry occurs during the oxidation of foodstuffs in the tricarboxylic acid (TCA) cycle. Oxalosuccinic acid, one of the intermediates in this cycle, has a carbonyl group (in this case a ketone) b to one of its three carboxyl groups. only this carboxyl has a C=O beta to it . HOOC O a b COOH COOH Oxalosuccinic acid O HOOC COOH + CO 2 a-Ketoglutaric acid