UoB-CLN-LAB-QCD-010 Impact assessment of equipment
advertisement

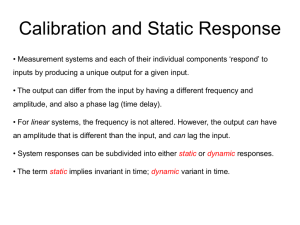
Impact assessment of equipment failure or equipment calibration failure Guideline Purpose and use of this guideline document On occasion equipment used for the processing, analysis or storage of clinical trial samples will fail, or will fail a calibration check. The impact on sample integrity and analysis must be assessed and documented. This document provides guidelines for how to carry out this assessment and may be used to develop trial-specific procedures. Scope of this guideline document This document provides guidelines for assessing the impact of equipment failure or calibration failure on sample integrity and analysis. It also references other guideline documents and example forms which it may be necessary to consider depending upon the circumstances. Record keeping The impact of equipment failure or calibration failure on sample integrity and analysis must be assessed and documented. An example form is available (see UoB-CLN-LAB-QCD-029 Impact assessment of equipment failure or equipment calibration failure report). A number of other records pertaining to equipment failure, analytical failure and adverse event reporting may also be completed (see references within Guidelines section below). All records must be stored safely and securely throughout the trial and archived with the other trial documents when the trial closes. Guidelines Any equipment failure should be acted upon and recorded. o Consider completing an adverse event report (see UoB-CLN-LAB-QCD-016 Adverse Events in the analytical laboratory and UoB-CLN-LAB-QCD-032 Adverse Event report). o Equipment failure should be documented along with any repairs (see UoB-CLN-LABQCD-002 Equipment maintenance and calibration checks and UoB-CLN-LAB-QCD-021 Equipment maintenance record). o If the equipment failure involves a storage unit then follow trial-specific procedures (see UoB-CLN-LAB-QCD-019 Procedures following failure of a storage unit). o If the equipment failure causes failure of an analytical run then document this separately (see UoB-CLN-LAB-QCD-009 Analytical assay failure and UoB-CLN-LAB-QCD-028 Analytical failure report). Assess the impact of the equipment failure or equipment calibration failure by evaluating and commenting on the following: o The type of equipment affected. o The magnitude of the calibration failure. o The elapsed time between the previous calibration date and the date of calibration check failure. o The analyses conducted using the affected equipment during the period above. o The effect of the failure on the accuracy and precision of the analytical results. o Any other relevant factors such as integrity of samples and reagents. Document this impact assessment (for an example form see UoB-CLN-LAB-QCD-029 Impact assessment of equipment failure or equipment calibration failure report) and consider having the report reviewed and signed off appropriately. UoB-CLN-LAB-QCD-010 Impact assessment of equipment failure or equipment calibration failure v1.0 (EAv1.0) Page 1 of 2 Impact assessment of equipment failure or equipment calibration failure Guideline Related documents UoB-CLN-LAB-SOP-001 Procedures for GCP compliance in the laboratory UoB-CLN-LAB-QCD-002 Equipment maintenance and calibration checks UoB-CLN-LAB-QCD-009 Analytical assay failure UoB-CLN-LAB-QCD-016 Adverse events in the laboratory UoB-CLN-LAB-QCD-019 Procedures following failure of a storage unit UoB-CLN-LAB-QCD-021 Equipment maintenance record UoB-CLN-LAB-QCD-028 Analytical failure report UoB-CLN-LAB-QCD-029 Impact assessment of equipment failure or equipment calibration failure report UoB-CLN-LAB-QCD-032 Adverse event reporting UoB-CLN-LAB-QCD-010 Impact assessment of equipment failure or equipment calibration failure v1.0 (EAv1.0) Page 2 of 2